Tag: FDA
The globalization of aortic innovation: A cautionary tale of U.S. regulatory...
In 1954, Dr. Michael DeBakey transformed vascular surgery with a homemade Dacron graft sewn on his wife’s sewing machine. His spirit of innovation laid...
Appeal filed over ‘not-approvable’ letter for breakthrough venous valve replacement
Venous valve developer enVVeno Medical has announced that it will file a request for supervisory appeal of the "not-approvable" letter from the Center for...
FDA begins ‘real-time’ reporting of adverse event data
The Food and Drug Administration (FDA) recently announced that it has begun daily publication of adverse event data from the FDA Adverse Event Reporting...
Boston Scientific recalls Carotid Wallstent Monorail devices over ‘manufacturing defect’
Boston Scientific has recalled its Carotid Wallstent Monorail endoprosthesis owing to a “manufacturing defect” that has led to devices having an inner lumen that...
FDA grants approval for IDE study on Advance Evero 18 everolimus-coated...
The Food and Drug Administration (FDA) has granted approval for Cook Medical to initiate an investigational device exemption (IDE) study on the Advance Evero...
FDA deems Envveno’s VenoValve ‘not-approvable’
The Food and Drug Administration (FDA) has issued a letter to Envveno Medical stating that its VenoValve technology is "not-approvable," a company press release...
Vivasure Medical submits premarket approval to FDA for PerQseal Elite closure...
Vivasure Medical has announced the submission of a premarket approval application to the Food and Drug Administration (FDA) for its PerQseal Elite vascular closure...
Senior vascular surgeons: 10 options beyond the operating room
As vascular surgeons advance in their careers, many begin to consider alternative professional paths that draw upon their clinical expertise, while offering greater flexibility,...
Gore announces FDA approval and first commercial implant of large-diameter thoracic...
Gore recently announced the expansion of the Gore Tag conformable thoracic stent graft with Active Control system product line, following Food and Drug Administration...
FDA announces plans to address medical device shortage risks
The Food and Drug Administration (FDA) has issued a statement outlining measures to enhance protections against medical device shortages.
According to Michelle Tarver, director...
When RCTs are essential for novel vascular access device regulation
As part of a 2024 VEITHsymposium (New York City; Nov. 19–23) debate, Robert E Lee, MD, a medical officer recently retired from the Food...
Survey of off-label treatment of complex repair pinpoints factors behind underreported...
The potential for cost and time to be key factors in low levels of outcomes data reporting emerged during a national survey of the...
Acellular tissue-engineered vessel granted FDA Regenerative Medicine Advanced Therapy designation for...
Humacyte recently announced it has been granted Regenerative Medicine Advanced Therapy (RMAT) designation from the US Food and Drug Administration (FDA) for its investigational...
Gore announces first commercial implant of Gore Tag thoracic branch endoprosthesis...
Gore has announced the first commercial use of the Gore Tag thoracic branch endoprosthesis (TBE) in Canada.
The news came as Canadian government health authority...
Vascular community has a role to play in ensuring safety of...
Physician-sponsored Investigational Device Exemption (IDE) trials have an important role to play in the continued development and refinement of aortic endovascular devices, attendees of...
Analysis will kickstart conversation on F/BEVAR outcomes
New data presented during Friday's Plenary Session 6 (10–11 a.m. in the West Building, Skyline Ballroom) will shed light on trends in the adoption...
Getinge and Cook Medical enter US commercial distribution agreement for iCast...
Getinge and Cook Medical today announced an exclusive sales and distribution agreement for the iCast covered stent system, which recently received Food and Drug...
FORS-powered LumiGuide 3D imaging technology is rolled out at specialized centers...
Andres Schanzer, MD, has hailed it "one of the most exciting changes" seen in imaging during the course of his career. Philips’ LumiGuide "human...
Breakthrough Device designation granted for Efemoral scaffold system designed to treat...
Efemoral Medical has announced the granting of Food and Drug Administration (FDA) Breakthrough Device status for its novel Efemoral vascular scaffold system (EVSS) designed...
Viabahn VBX stent graft receives FDA approval
Gore has announced recent Food and Drug Administration (FDA) approval of a lower profile Viabahn VBX balloon expandable endoprosthesis (VBX stent graft) for the treatment...
First patient treated in ARISE II study of ascending stent graft
Gore has announced the first patient implantation of the company's ascending stent graft in the ARISE II trial, describing this as an exciting step...
Humacyte submits Biologics License Application to FDA seeking approval of Human...
Humacyte today announced that it has submitted a Biologics License Application (BLA) to the Food and Drug Administration (FDA) seeking approval of the Human...
VOYAGER PAD analyses shed light on use of rivaroxaban in high-risk...
New analyses from the VOYAGER PAD clinical trial in both high-risk and fragile patients and those with and without comorbid coronary artery diseases (CAD)...
TCT 2023: First presentation of complete patient-level dataset on paclitaxel and...
Data from a patient-level meta-analysis—a factor in the Food and Drug Administration’s (FDA) recent change of position on the use of paclitaxel-coated devices to...
Enrollment of first patient in Neuroguard carotid stent system trial announced
Contego Medical has announced enrollment of the first patient in the prospective, multicenter PERFORMANCE III trial aimed at further evaluating the safety and effectiveness of the...
VasQ AVF creation device receives FDA clearance
Laminate Medical Technologies has announced their flagship device, the VasQ external vascular support, has been cleared by the Food and Drug Administration (FDA) for...
Endovascular stablization system for infrarenal AAAs gains FDA Fast Track designation
Nectero Medical announced that the Food and Drug Administration (FDA) has granted Fast Track designation for the Nectero Endovascular Aneurysm Stabilization Treatment (Nectero EAST)...
Light-activated drug-coated balloon granted FDA approval for clinical study
Alucent Biomedical has announced that the Food and Drug Administration (FDA) granted an investigational device exemption (IDE) for a U.S. clinical study of AlucentNVS,...
Vascular Specialist–August 2023
In this issue:
SVS responds to New York Times exposé on overuse of vascular interventions
Long-awaited FDA update finds data do not support excess...
‘FDA’s mission to bring safe and effective vascular surgery devices to...
The Vascular and Endovascular Devices Team (VEDT) in the Food and Drug Administration (FDA) Office of Cardiovascular Devices appreciates the Vascular Specialist editorial board's...
Comments sought on CMS proposed coverage expansion for carotid stenting
SVS members are encouraged to submit comments—due Aug. 10—to the Centers for Medicare & Medicaid Services (CMS) on its proposed national coverage determination on...
Endologix announces first patients treated with the Detour system
Endologix has shared in a press release that the first patients underwent percutaneous transmural arterial bypass (PTAB) using the Detour system since Food and Drug...
Long-awaited FDA update finds data do not support excess mortality risk...
In a letter to healthcare providers dated July 11, 2023, the Food and Drug Administration (FDA) communicates that the risk of mortality associated with...
Surmodics receives FDA approval for the SurVeil drug-coated balloon
Surmodics has announced the receipt of Food and Drug Administration (FDA) approval for the SurVeil drug-coated balloon (DCB).
A company press release notes that the...
FDA approves study of ZFEN+ for treatment of aortic aneurysms
The Food and Drug Administration (FDA) has granted approval for Cook Medical to initiate an investigational device exemption (IDE) study on the Zenith fenestrated+...
Two-year DETOUR2 study results presented at VAM 2023
Endologix has announced the 24-month results of the DETOUR2 study, presented at VAM 2023, the annual meeting of the Society for Vascular Surgery (SVS),...
FDA seeks to ‘modernize’ clinical trials with new draft guidance
The Food and Drug Administration (FDA) has released draft guidance with updated recommendations for good clinical practices (GCPs) aimed at modernizing the design and...
On 45th anniversary, CX Symposium chairman credits Chicago vascular surgical tandem...
The occasion of a seminal meeting held in Chicago by former Society for Vascular Surgery (SVS) Presidents John J. Bergan, MD, and James S.T....
Getting medical devices to market: The future might not be now,...
The development of medical devices in the vascular space faces increasing challenges amid moves afoot at the Food and Drug Administration (FDA), according to...
Surmodics provides regulatory update related to FDA premarket approval application for...
Surmodics recently announced it has received a letter from the Food and Drug Administration (FDA) related to its premarket approval (PMA) application for the...
Bentley launches rebranded crossing catheter
Germany-based endovascular device company Bentley announced the launch of the BeBack crossing catheter—formerly known as the GoBack—designed for the treatment of heavily calcified lesions...
Penumbra launches Lightning Flash mechanical thrombectomy system
Penumbra have announced the Food and Drug Administration (FDA) clearance and launch of its Lightning Flash mechanical thrombectomy system.
“Lightning Flash features Penumbra’s novel Lightning intelligent...
Endologix receives FDA approval of PMA supplement for AFX2 system
Endologix recently announced that it has received Food and Drug Administration (FDA) approval for a premarket approval (PMA) supplement relating to the AFX2 system.
According...
Eastern Vascular gathering challenged to forge next generation of evolution
Evolution was the core theme of the Presidential Address Robert Rhee, MD, delivered from the Eastern Vascular Society (EVS) annual meeting stage in Philadelphia...
Chocolate Touch DCB for treatment of peripheral arterial disease receives FDA...
Genesis MedTech Group announced that the Food and Drug Administration (FDA) has approved the Chocolate Touch drug-coated balloon (DCB) percutaneous transluminal angioplasty (PTA) catheter...
Amplifi vein dilation system continues to show promise following latest first-in-human...
An update from the first-in-human study assessing the Amplifi vein dilation system (Artio Medical) was delivered by Surendra Shenoy, MD, from Washington University School of...
Terumo Aortic announces new technology add-on payment for Thoraflex Hybrid device...
The Centers for Medicare and Medicaid Services (CMS) has granted approval of a new technology add-on payment (NTAP) for Terumo Aortic’s Thoraflex Hybrid device—used...
No time like the present: The moral imperative for advocacy in...
“I’m so sorry but we have to cancel your surgery”—10 words any warm-blooded surgeon dreads uttering. This time around, it was not for the...
TCAR: First patient enrolled in ROADSTER 3 post-approval study
Silk Road Medical has announced enrollment of the first patient in ROADSTER 3, which the company claims is the the first prospective, multicenter, single-arm...
AngioDynamics announces FDA clearance of expanded indications for Auryon atherectomy system
AngioDynamics recently announced receiving Food and Drug Administration (FDA) 510(k) clearance of an expanded indication for the Auryon atherectomy system to include arterial thrombectomy.
The FDA recently...
Selution SLR receives second FDA IDE approval
Selution SLR, MedAlliance’s sirolimus-eluting balloon, has received conditional Food and Drug Administration (FDA) investigational device exemption (IDE) approval to initiate its pivotal clinical trial...
Enrollment begins in PERSEVERE clinical trial of hybrid prothesis for aortic...
Artivion announced today that it has initiated enrollment in the PERSEVERE clinical trial to determine if patients with an acute DeBakey Type I aortic dissection...
Cordis announces start of enrollment in RADIANCY clinical study in Europe
Cordis has announced the start of the European RADIANCY premarket clinical study of a stent system designed for radial access in the treatment of...
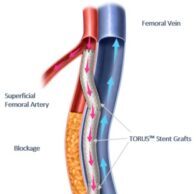
AVeVA study confirms benefit of covered stent placement in graft-vein anastomotic...
A prospective, multicenter study involving the Covera vascular covered stent (BD) has confirmed the benefits of immediate, post-percutaneous transluminal angioplasty (PTA) placement of the...
PAD: Ra Medical Systems receives FDA 510(k) clearance for the Dabra...
Ra Medical Systems has announced receipt of Food and Drug Administration (FDA) 510(k) clearance for the company’s Dabra 2.0 catheter as part of the...
Terumo Aortic announces first commercial implant of Thoraflex Hybrid in US
Following the recent approval by the Food and Drug Administration (FDA) of the Thoraflex Hybrid frozen elephant trunk (FET) device for the treatment of...
Expanded Medicare coverage for TCAR in standard surgical risk patients...
Silk Road Medical today announced that the Centers for Medicare and Medicaid Services (CMS), through collaboration with the Society of Vascular Surgery's Patient Safety...
FDA grants IDE approval for Selution SLR drug-eluting balloon
MedAlliance’s Selution SLR drug-eluting balloon (DEB) has received investigational device exemption (IDE) approval from the Food and Drug Administration, making it the first limus...
New long-term data of paclitaxel devices continue to show no increased...
New long-term data from the SAFE-PAD (Safety assessment of femoropopliteal endovascular treatment with paclitaxel-coated devices) study were presented today as late-breaking clinical research at...
Venovo venous stent returns to US market after 2021 recall
BD recently announced that its Venovo venous stent is back on the U.S. market following a recall last year.
In 2019, the company reported that the...
FDA approves expanded indications for TCAR’s Enroute stent
The Food and Drug Administration (FDA) approved expanded indications for the Enroute stent system—part of the transcarotid artery revascularization (TCAR) procedure—to include patients at...
Terumo Aortic announces FDA approval for Thoraflex Hybrid frozen elephant trunk...
Terumo Aortic today announced that the Food and Drug Administration (FDA) has granted approval of the Thoraflex Hybrid frozen elephant trunk device for commercial...
Shockwave Medical announces global launch of new peripheral IVL catheter
Shockwave Medical has announced the global commercial availability of the Shockwave M5+ peripheral intravascular lithotripsy (IVL) catheter after receiving both Food and Drug Administration (FDA) and...
Cook Medical receives FDA Breakthrough Device designation for Zenith Thoraco+ endovascular...
Cook Medical’s Zenith Thoraco+ endovascular system (Thoraco+) has received Breakthrough Device designation from the Food and Drug Administration (FDA), a press release reports.
The company...
Database analysis findings ‘matched precisely’ with reported reasons for venous stent...
A recent analysis recalled Venovo and Vici venous stents—initiated last year—characterized “significant differences” between reported device and patient issues, and subsequent interventions for the...
Ra Medical Systems receives FDA approval for increased enrollment in atherectomy...
Ra Medical Systems, a medical device company focused on developing the excimer laser system to treat vascular diseases, has announced that enrollment has reached...
FDA issues guidance documents for including patient perspectives in medical device...
The Food and Drug Administration (FDA) has issued two final guidances providing recommendations for including patient perspectives in medical device clinical studies.
As per...
Cook Medical receives FDA breakthrough designation for new drug-eluting stent
Cook Medical has received Breakthrough Device designation from the Food and Drug Administration (FDA) on a new drug-eluting stent (DES) for below the knee...
Philips integrates cloud-based AI and 3D mapping into its mobile C-arm...
Royal Philips has announced physicians will now have access to advanced new 3D image guidance capabilities through its image-guided therapy mobile C-arm system—Zenition. The...
Our forever plague
The following is a combined and revised version of the November and December 2021 editorials on fake news and science denial published in Vascular...
FDA issues updated safety communication on use of Endologix AFX endovascular...
The Food and Drug Administration (FDA) has issued an updated safety communication on the use of Endologix AFX endovascular grafts. This update includes...
FDA approves two pediatric venous thromboembolism indications for rivaroxaban
The Food and Drug Administration (FDA) has approved two pediatric indications for Xarelto (rivaroxaban): the treatment of venous thromboembolism (VTE) and reduction in the risk of recurrent...
Envveno Medical reports successful completion of first VenoValve surgery in US...
Envveno Medical—formerly Hancock Jaffe Laboratories—recently announced that the first VenoValve surgery in the company’s SAVVE US pivotal trial for the VenoValve has been successfully...
Sanford invents Breakthrough Device for TAAA
An investigational device invented at Sanford Health in Sioux Falls, South Dakota, that helps high-risk vascular disease patients has been granted a Breakthrough Device...
Terumo Aortic announces first commercial implants of RelayPro in US
Terumo Aortic today announced the first commercial implants in the U.S. of its RelayPro thoracic stent-graft system. This follows the recent approval by the...
‘Tremendous advances in imaging possibilities’ signal further innovation, says leading vascular...
It was a complex repair in the thoracoabdominal region of the aorta around 10 years ago, and Matthew Eagleton, MD, and his surgical team...
FDA approves expanded PAD indication for rivaroxaban plus aspirin
The Janssen Pharmaceutical Companies of Johnson & Johnson today announced that the Food and Drug Administration (FDA) has approved an expanded peripheral arterial disease...
PRISTINE registry with Selution SLR sirolimus drug-eluting balloon completes enrollment
MedAlliance has announced completion of patient enrollment in the PRISTINE clinical trial with the Selution SLR 018 drug-eluting balloon (DEB) for the treatment of patients with below-the-knee...
RelayPro thoracic stent graft approved by FDA
Terumo Aortic today (Friday, Aug. 6) announced that the Food and Drug Administration (FDA) has granted approval of the RelayPro thoracic stent-graft system for...
VenoValve granted FDA Breakthrough Device designation
Hancock Jaffe Laboratories today (Wednesday, Aug. 4) announced that the Food and Drug Administration (FDA) has granted Breakthrough Device Designation status to the company's...
Surmodics announces successful first patient use of Pounce thrombectomy system
Surmodics has announced that J. Michael Bacharach, MD, a vascular interventionalist/cardiologist at North Central Heart, a division of Avera Heart Hospital in Sioux Falls,...
Surmodics builds thrombectomy portfolio with acquisition of Vetex Medical
Surmodics recently announced that it has acquired privately-held Vetex Medical Limited, expanding the company's thrombectomy portfolio with a second Food and Drug Administration (FDA) 510(k)-cleared...
Boston Scientific recalls Vici SDS and Vici RDS venous stent systems...
According to a medical device recall notice posted on the Food and Drug Administration (FDA) website, Boston Scientific has recalled its Vici venous stent...
ACC.21: SAFE-PAD finds no increased risk of death with drug-coated devices
Researchers have found no statistically significant difference in mortality between patients treated with drug-coated devices and non-drug-coated devices in the SAFE-PAD study. Eric Secemsky,...
VenoValve: Chronic venous insufficiency device gains brisk double of first US...
Hancock Jaffe Laboratories recently revealed that the United States Patent and Trademark Office (USPTO) issued the first patent covering the company’s VenoValve. The patent is...
US surgeons begin commercial use of Gore Excluder conformable AAA endoprosthesis...
W. L. Gore & Associates (Gore) today announced the first use of the Food and Drug Administration (FDA)-approved Gore Excluder conformable abdominal aortic aneurysm...
FDA grants breakthrough device designation for Zenith fenestrated+ endovascular graft
The Zenith fenestrated+ endovascular graft (ZFEN+) product (Cook Medical) has received breakthrough device designation from the Food and Drug Administration (FDA). This designation is...
Medtronic recalls unused Valiant Navion thoracic stent graft system
Medtronic has voluntarily issued a global recall of its Valiant Navion thoracic stent graft system and informed physicians to immediately cease use of the...
Postmarket study of sirolimus-eluting balloon enrolls first patient
MedAlliance has announced enrollment of the first patient in SUCCESS PTA, its large post-market study with the drug-eluting balloon Selution SLR for the treatment...
Treo IDE primary endpoint results announced
In the wake of approval by the Food and Drug Administration (FDA) of the Treo abdominal aortic stent-graft system for the treatment of patients...
Fluoroquinolones linked to increased incidence of aortic aneurysms in US adults
Fluoroquinolones—one of the most commonly prescribed antibiotic classes in the United States—were associated with increased incidence of aortic aneurysm formation in U.S. adults, a...
FDA representatives respond to SWEDEPAD interim analysis, highlight need for continued...
Representatives from the Food and Drug Administration (FDA) referenced "important and reassuring" results from the recent interim analysis of the SWEDEPAD clinical trial in which...
Vesper Medical announces first enrollment in the VIVID trial
Vesper Medical recently announced initiation of its Food and Drug Administration (FDA) investigational device exemption (IDE) study, VIVID (Venous stent for the iliofemoral vein investigational clinical trial...
Janssen applies to FDA for new indication to expand use of...
The Janssen Pharmaceutical Companies of Johnson & Johnson announced today it has submitted a supplemental New Drug Application (sNDA) to the Food and Drug...
‘Nearly nothing’ in surgery will go untouched by AI, ACS Clinical...
Almost nothing will go untouched by artificial intelligence (AI) in healthcare and surgery, notably in the quickly evolving world of vascular and endovascular surgery,...
Vascular surgeons encouraged to consult talking points document on paclitaxel devices
Vascular surgeons are being encouraged to take consideration of a set of talking points about the risks and benefits of paclitaxel-equipped devices—which has been...
Terumo Aortic gains FDA approval for Treo endovascular device
Terumo Aortic has been granted Food and Drug Administration (FDA) approval for the Treo abdominal aortic stent-graft system to be sold in the U.S.
The...
First US commercial use of Tack Endovascular System in BTK arteries...
Intact Vascular has announced the first commercial use of its Tack Endovascular System (4F) in multiple sites across the United States.
Notably the first Food...
FDA grants Nexus aortic arch stent graft system breakthrough designation
Endospan was recently granted breakthrough device designation from the Food and Drug Administration (FDA) for the Nexus aortic arch stent graft system.
The FDA’s breakthrough...
FDA official delivers government agency’s early reaction to latest contentious meta-analysis...
HOLLYWOOD, Fla.—A Food and Drug Administration (FDA) official delivered a talk which represents the government agency’s earliest public reaction to the latest meta-analysis suggesting...
Early adaptation of SVS, STS TBAD joint document will drive robust...
The Society for Vascular Surgery (SVS) and the Society of Thoracic Surgeons (STS) recently published a joint document on reporting standards for type B...
Newly FDA-approved device for central venous occlusions hailed as exciting advance
HOUSTON—These are exciting times in the theater of dialysis access, expert in the field Eric Peden, MD, mused in the latter part of last...
Deploying robust, detailed SVS VQI data to help better define role...
In December 2018, the Journal of the American Heart Association published “Risk of death following application of paclitaxel‐coated balloons and stents in the femoropopliteal...
FDA keeps close watch on heparin dosing reports
The Food and Drug Administration (FDA) is closely monitoring reports of higher than usual doses of heparin being required to achieve activated clotting times...
SVS president presents point-by-point defense of Society position on paclitaxel controversy
NEW YORK—Kim Hodgson, MD, president of the Society for Vascular Surgery, delivered a robust defense of the SVS position on the drug-coated balloon (DCB)...
Regulatory unity can help drive medical device adoption globally, FDA official...
LAS VEGAS—Efforts at international collaboration across borders can drive harmony among disparate regulatory requirements and lead to greater global cooperation at the medical device...