Biotronik has announced the Food and Drug Administration (FDA) 510(k) clearance and CE mark of its Oscar (One Solution: Cross. Adjust. Restore) multifunctional peripheral catheter.
The company notes in a press release that Oscar is intended for percutaneous transluminal interventions in the peripheral vasculature. The device was developed to provide support during access into and to dilate stenoses in femoral, popliteal and infrapopliteal arteries.
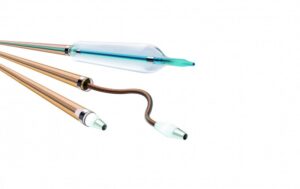
The Oscar peripheral multifunctional catheter is comprised of three user-adjustable components:
- Oscar support catheter with integrated Lock Grip
- Oscar extendable dilator
- Oscar length-adjustable percutaneous transluminal angioplasty (PTA) balloon
The Oscar system will be available in 11 total size configurations with either 0.014″/4Fr or 0.018″/6Fr guidewire/introducer sheath compatibility, Biotronik states. Additional standalone Oscar PTA balloons are also available separately to be used with the Oscar support catheter.
The company details that the Oscar support catheter and dilator are used in tandem to enable lesion access and crossing with a compatible guidewire, offering a wide range of support strength, adjusted with the extension of the dilator. At its strongest support level, Biotronik claims, the combination provides up to 55% more pushability than the leading 0.035″ support catheter.
The first procedure with the Oscar system in the USA was performed by Jihad A Mustapha, MD, president and chief executive officer and director of endovascular interventions at Advanced Cardiac & Vascular Centers for Amputation Prevention Grand Rapids, Michigan. “Oscar is a game-changing product. In cases where we had previously failed with multiple treatment approaches, the Oscar catheter made it possible to succeed, while also saving time and reducing how long the patient needs to spend on the table. This is one of the best support systems I have ever used, and the length-adjustable balloon functionality is exceptional,” stated Mustapha.
More than 70 cases have been performed in U.S. hospitals with the Oscar device as part of Biotronik’s pre-launch evaluations. Despite highly complex disease, the Oscar catheter demonstrated a 90% crossing success rate and a PTA technical success rate of 95%, the company reveals. In some cases, physicians had previously tried and failed with traditional crossing devices and strategies.
Biotronik advises that the product will be commercially available in the U.S. starting in the Spring of 2023 and in CE mark accepting regions in the second half of 2023.