BioGenCell has announced that it is recruiting for an international clinical trial to assess the company’s stem cell-based therapy for chronic limb-threatening ischemia patients who are at risk for limb amputation.
The clinical trial is currently taking place in medical centres in Israel, Europe, and the US, a press release details.
The company notes that the news follows a recent investment round that brought in $16 million, led by the entrepreneur Marius Nacht, as well as FDA and Health Authorities approvals in Israel and Europe to carry out the study.
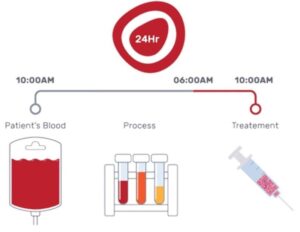
BioGenCell states that the company previously demonstrated the efficacy of their treatment in the prevention of limb amputation based on cells from patients’ blood in lab settings and in animal model, and reported promising results in a phase I clinical trial.
The company claims that their technology “transforms the patient’s self-blood cells to cells with rehabilitative functions”. These personal cells are then injected back into the patient, directly into the affected limb, where they encourage the generation of new, healthy blood vessels. By regenerating the blood flow to the limb, the therapy “potentially prevents limb amputation, or even death, and allows the patients to resume normal activity,” they write in the press release.
Yael Porat, founder and CEO of BioGenCell, commented on the company update: ”This is great news for patients with ischemic vascular diseases who are at risk of limb amputation.”
Shlomo Baytner of Laniado Hospital in Netanya, Israel, remarked: “BioGenCell’s innovative treatment is intended for patients who we can longer offer the option of catheterization or bypass. These patients suffer constant pain over a long period of time, and I am optimistic and hope that we will finally have a treatment available that can help these patients.”












