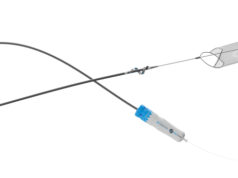
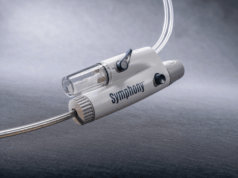
Surmodics has announced that it has received Food and Drug Administration (FDA) 510(k) clearance for its Pounce XL thrombectomy system.
Surmodics has announced that it has received Food and Drug Administration (FDA) 510(k) clearance for its Pounce XL thrombectomy system.
The Pounce system is indicated for the non-surgical removal of thrombi and emboli from the peripheral arterial vasculature in vessels 5.5–10mm in diameter, making it suitable for iliac, femoral and other arteries within this range. The Pounce XL thrombectomy system increases the size range of the Pounce platform, which also includes the thrombectomy system, indicated for 3.5–6mm peripheral arteries, and the Pounce Low Profile (LP) thrombectomy system, indicated for 2–4mm peripheral arteries. The Pounce thrombectomy system and Pounce LP thrombectomy system were introduced in 2021 and 2024, respectively.
Surmodics expects to initiate limited market release for the Pounce XL thrombectomy system in the first half of 2025, with commercialization planned following the completion of the limited market release.