Ellen Dillavou, MD, division chief of vascular surgery at WakeMed Heart Center in Raleigh, North Carolina, primary investigator (PI) for the VenoStent trial, presented new data during the 2024 VEITHsymposium in New York City (Nov. 19–23) about the SelfWrap (VenoStent) bioabsorbable perivascular wrap, outlining how the novel device can, she says, improve arteriovenous fistula (AVF) maturation and patency.
According to data published in the Journal of the American Society of Nephrology (JASN) and American Journal of Kidney Disease (AJKD), annually, 5 million patient lives are put at risk by 60% one-year failure rates of AVFs and arteriovenous grafts (AVGs), as well as 20% one-year failure rates of vein grafts in bypass grafting, she said.
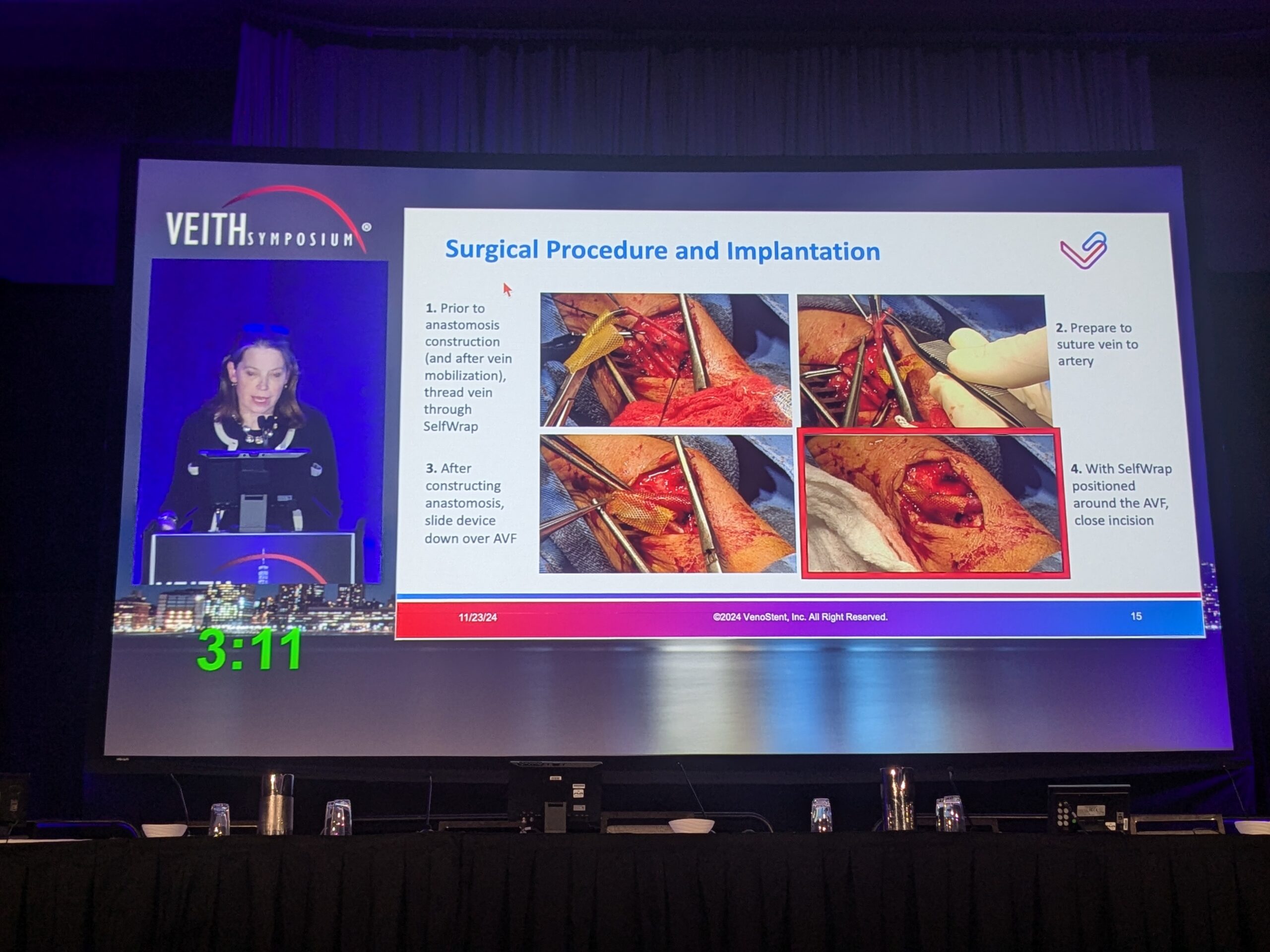
One solution, Dillavou argued, is the SelfWrap device. Receiving its Food and Drug Administration (FDA) Breakthrough Device designation in May 2022, followed by investigational device exemption (IDE) approval in May 2023, this vascular wrap made of bioabsorbable polymers uses an artery-like mechanical support to help veins first “behave” and then “become” like an artery. It also regulates flow—which Dillavou stated will “impart hemodynamic benefits.”
“In every large animal model which tested AVFs, AVGs and bypass grafts over five years and three different centers, the advanced materials approach that ‘arterializes’ veins significantly reduces neointimal hyperplasia,” said Dillivou.
The SelfWrap trial’s objective was to demonstrate feasibility and evaluate the safety and performance of the SelfWrap device by enrolling 20 participants in a single-center, prospective, single-arm study, with follow-up obtained at six months up to 60 months. The primary endpoint was a high patency rate. Three SelfWrap sizes were employed, with selection based on vein size. A total of 13 patents (65%) were given brachiocephalic fistulas (BCFs), two received basilic vein transpositions (BVTs), and five were given radiocephalic fistulas (RCFs).
Dillavou reported that there were no adverse events probably or definitely related to the device through 36 months, as well as no device deficiencies nor adverse device effects.The SelfWrap device had a high maturation rate, she reported. Functional maturation was 95%, compared to the <58% expected, and unassisted maturation was 90% for SelfWrap, compared to 33%. Further, SelfWrap saw 89.5% of catheters removed at nine months, 94% of which were unassisted, compared to ~27% at nine months for untreated AVFs. Dillavou noted that, should the results translate to the ongoing IDE study, they would prove impactful in the space.












