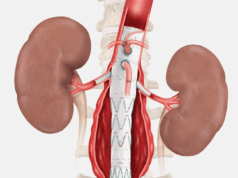
Gore today announced CE-mark approval of an expanded indication for the Gore Viabahn VBX balloon-expandable endoprosthesis (VBX stent graft) when used as a bridging stent with branched and fenestrated aortic endografts in the treatment of aortic aneurysms involving the renal and mesenteric arteries.
“This is a landmark indication for a stent graft, equipping physicians with an on-label solution for more patients in complex pathologies,” said Luca Bertoglio (Spedali Civili Brescia, Brescia, Italy), co-ordinating investigator of the EMBRACE registry.
A total of 259 patients were enrolled in EMBRACE. Gore reports high rates of patency and freedom from endoleaks, target vessel instability and reinterventions were observed with the VBX stent graft in both branched and fenestrated endovascular aneurysm repair (BEVAR and FEVAR) cohorts at one year.
“We have studied performance in both fenestrated and branched cohorts, and the results demonstrate that we can safely and effectively treat these patients with the VBX device,” Bertoglio added.
This multicentre, retrospective and prospective registry was conducted to evaluate the clinical performance and safety of the VBX stent graft as a bridging stent. A total of 14 centres in Europe enrolled 259 patients who have had treatment with the VBX stent graft. These patients will have prospective follow-up visits up to five years after the index procedure.
“Physicians have waited some time for an approved bridging stent indication for fenestrated and branched repair. Having a CE-marked indication is very important, as it is certainly of relevance to the endovascular community and the eligible patient population,” said Mauro Gargiulo (IRCCS S Orsola Hospital and University of Bologna, Bologna, Italy), principal investigator of the EXPAND registry. “I applaud Gore for recognising the need for a CE-mark indication and for being committed to conducting such thorough and expertly run clinical studies like EXPAND and EMBRACE.”
In the coming weeks, hospitals will start implanting the newly indicated VBX stent graft in BEVAR and FEVAR procedures. The official launch is planned after summer when many European and local congresses are taking place.
Gore shares that the VBX stent graft offers precise delivery and supports positive outcomes in a variety of complex applications. The device was developed utilising the small diameter, ePTFE stent graft technology from the Gore Viabahn endoprosthesis. The VBX stent graft is available in a range of diameters from 5 to 11mm and lengths of 15, 19, 29, 39, 59 and also 79mm. It is currently the longest balloon-expandable stent graft available, according to Gore, covering a wide variety of treatment needs. The company adds that the VBX stent graft offers the largest range of diameter adjustability in a single device, with a maximum post-dilated diameter of up to 16mm with 8L or 11mm devices.