Endologix recently announced that the Centers for Medicare & Medicaid Services (CMS) has granted a Transitional Pass-Through (TPT) payment for the Detour system, effective since 1 January 2024.
The TPT payment was created to facilitate patient access for qualifying new medical technologies that substantially improve the diagnosis or treatment of Medicare beneficiaries. TPT will provide hospitals with additional device reimbursement when the DETOUR system is used for eligible cases in the hospital outpatient setting. Details on the TPT, code C1604, can be found in the January 2024 Update of the Hospital Outpatient Prospective Payment System (OPPS), accessible on the CMS website and the Endologix website.
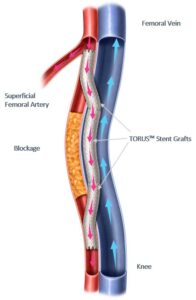
Percutaneous transmural arterial bypass (PTAB) with the Detour system, a US Food and Drug Administration (FDA) Breakthrough Device, received premarket approval in June 2023. Endologix states that PTAB offers a novel approach to treating complex peripheral arterial disease (PAD). The company details that Detour enables physicians to bypass lesions in the superficial femoral artery, by using conduits routed through the femoral vein via a transmural passage, to restore blood flow to the leg. According to Endologix, this approach is effective for patients with long lesions (20–46cm in length), those who have already undergone failed endovascular procedures, or those who may be suboptimal candidates for open surgical bypass.
“Being awarded the TPT designation marks an important milestone in our reimbursement strategy and the commercial launch of the Detour system for treating patients with complex PAD. At Endologix, our core mission is to deliver healthcare innovation to improve the lives of patients with vascular disease. With the support from CMS for qualified patients, we eagerly anticipate more patients to benefit from PTAB,” said Matt Thompson, president and CEO of Endologix.
The company advises that the Detour system is indicated for use for percutaneous revascularization in patients with symptomatic femoropopliteal lesions from 200–460mm in length with chronic total occlusions (100–425mm) or diffuse stenosis >70% who may be considered suboptimal candidates for surgical or alternative endovascular treatments. The Detour system, or any of its components, is not for use in the coronary and cerebral vasculature.












