The National Institutes of Health (NIH)-funded CREST-2 study has found that, for people with high-grade asymptomatic carotid artery stenosis who have not experienced recent stroke symptoms, a carotid artery stenting (CAS) procedure—combined with intensive medical therapy—significantly lowered stroke and death rates compared with medical therapy alone. The more traditional “gold standard” approach of carotid endarterectomy (CEA) did not show the same benefit, however.
These first “game-changing” results outlining four-year outcomes were presented at the 2025 VEITHsymposium in New York City (Nov. 18–22) by CREST-2 co-principal investigator Brajesh K. Lal, MD, a professor of surgery at the University of Maryland in Baltimore. Earlier the same day, the data were delivered at the 2025 Society of Vascular and Interventional Neurology (SVIN) annual meeting in Orlando, Florida (Nov. 19–22) by James Meschia, MD, a vascular neurologist at the Mayo Clinic in Jacksonville, Florida. The trial results were also published in the New England Journal of Medicine.
Lal outlined how the study’s two simultaneously-running randomized controlled trials (RCTs) comparing CAS plus medical therapy to medical management alone, and CEA plus medical therapy to medical management alone, enrolled 2,485 patients from 155 sites across five countries.
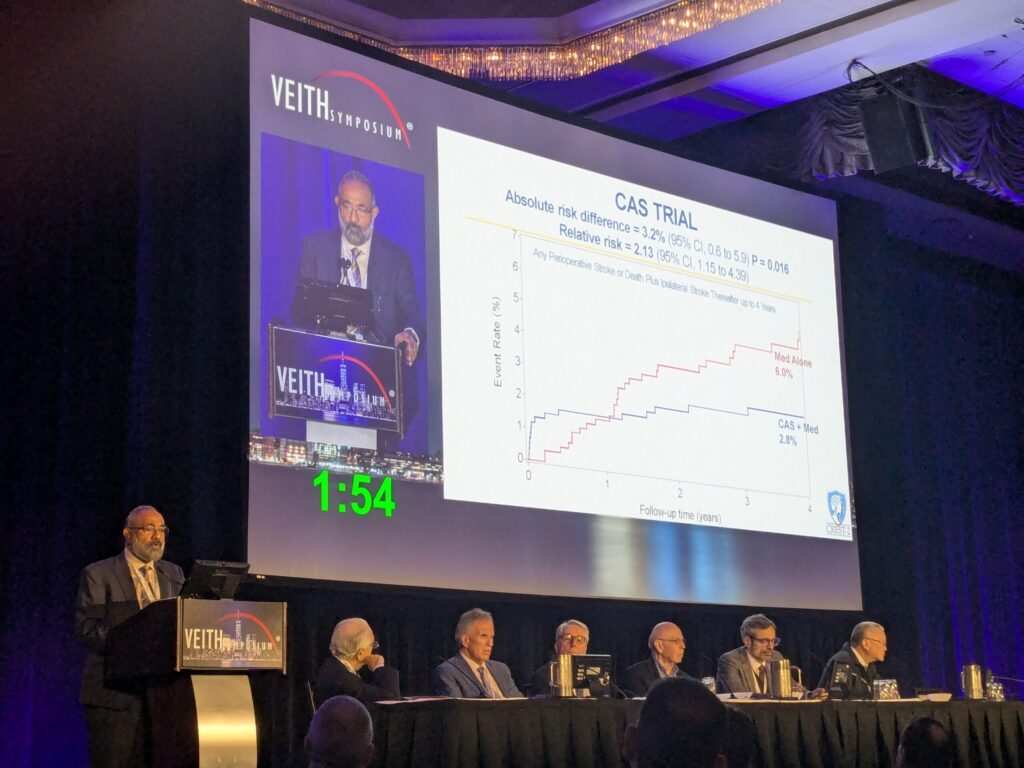
In the CAS trial, stroke and death rates out to four years were 6% in patients who were assigned medical management and 2.8% when CAS was added, Lal told VEITH 2025. “The absolute risk difference of 3.2% in favor of CAS was statistically significant,” he said.
In the CEA trial, stroke and death out to four years was 5.3% in the medical therapy group and 3.7% when CEA was added, Lal continued. “The absolute risk difference was still in favor of CEA; however, it did not reach significance.”
Lal explained: “The patterns of differences in the CAS and CEA trials were mirrored in the 44-day periprocedural period; however, in the post-procedural period starting 45 days out to four years, both CAS and CEA performed better in terms of preventing stroke and death compared to their respective medical management groups.”
Concluding, Lal emphasized how the absolute difference favoring CAS was significant, with “31 people with high-grade asymptomatic carotid stenosis needed to be treated to prevent a primary event at four years in the trial.”
Thomas Brott, MD, co-principal investigator and a professor of neurology at the Mayo Clinic College of Medicine in Jacksonville, Florida, followed Lal at the VEITH 2025 podium to tackle whether the CREST-2 evidence is conclusive or further study is needed.
“In one generation, since ACAS, we’ve gone from medical risk of 11% to a risk of 6%, which is remarkable, particularly in light [of the fact] that today our surveillance is via MRI [magnetic resonance imaging] in almost all instances,” he said.
“The trial shows CAS is effective, Brott continued, with its stroke and death rate “one half of medicine alone.” As for CEA, he said, “there is a difference, but it did not reach statistical significance.”
So, Brott asked, are future studies needed?
“Yes of course,” he said, going on to list several areas in need of scrutiny, among them carotid plaque risk, patients with higher risk features, carotid stent optimal design, flow reversal as well as the optimal regimen of medical therapy.
“The elephant in the room in one of these areas of further study is TCAR [transcarotid artery revascularization]: no level-1 evidence. But a randomized controlled trial showing a drop in risk that you would consider clinically significant over four years would require a sample size of 4,400 patients,” he added. “Discoveries will happen, but rock-solid validation may not be feasible via RCTs in patients with asymptomatic carotid disease.”