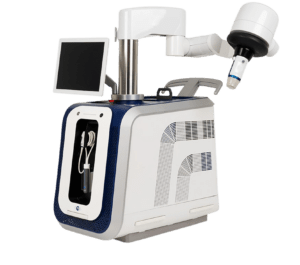
The Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) application for the VEINRESET multicenter pivotal study that will evaluate Sonovein high-intensity focused ultrasound (HIFU) treatment for varicose veins, it has been announced.
Antonios Gasparis, MD, director of the Center for Vein Care at Stony Brook University in Stony Brook, New York, recently reported the first U.S. assessment of the echotherapy platform, showing 100% technical feasibility at three months.
Sonovein treats primary insufficiency of great saphenous veins (GSVs) by concentrating therapeutic ultrasounds to an internal focal point from outside of the body, according to investigators.
The pivotal study will be conducted at four centers in the U.S. and Europe, with Steve Elias, MD, director of the Center for Vein Disease at Englewood Hospital in New Jersey, acting as principal investigator in the U.S.
“We believe that this key study will confirm the positive findings of the FDA feasibility study, completed just two months ago, and will ultimately allow us to commercially address the U.S. market,” said Yann Duchesne, executive chairman of Theraclion, the company behind Sonovein, in a press release.












