This advertorial is sponsored by Gore.
One mainstay endovascular aneurysm repair (EVAR) device is celebrating not just a quarter century of being commercially available, but the innovation it has inspired along the way. Michel Makaroun, MD, and Willy Davison, PhD, discuss the evolution and innovation of the GORE® EXCLUDER® device family over time.
Twenty-five years of patient impact and durability is worth celebrating in the life of any medical device. And when a device reaches this milestone, it is celebrating more than just 25 years in the treatment landscape. It is a legacy of making a difference for physicians and their patients.
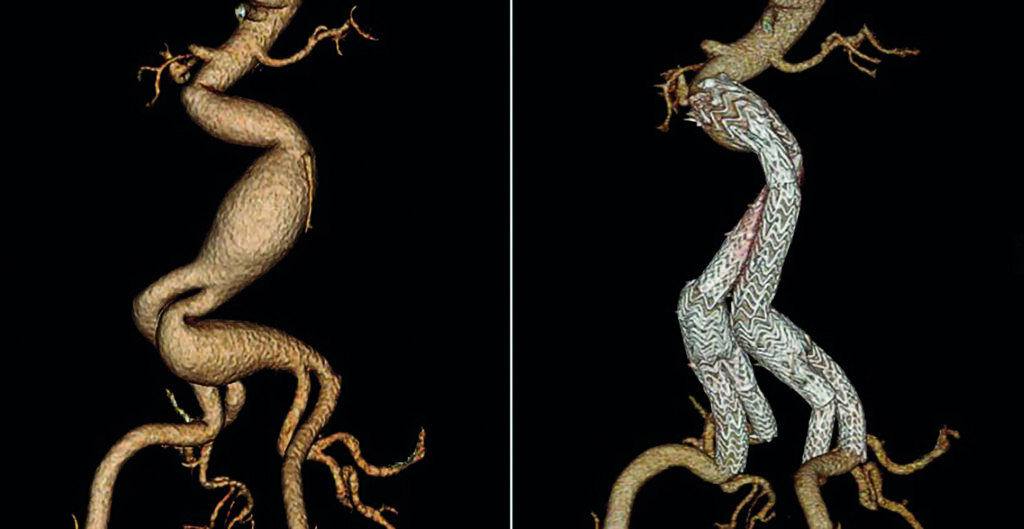
The GORE® EXCLUDER® AAA Endoprosthesis for abdominal aortic aneurysm (AAA) is commemorating 25 years of commercial availability since earning its CE mark in September 1997. It has been used to treat more than 440,000 patients worldwide* and has become the most-studied EVAR device according to company-sponsored trials and registries shown on ClinicalTrials.gov for currently available stent grafts.

“The EXCLUDER® AAA Endoprosthesis has been on the market helping patients for well over two decades: A truly remarkable accomplishment and the longest stretch in the industry,” said Makaroun, chief of the Division of Vascular Surgery at the University of Pittsburgh Medical Center and an investigator in each EXCLUDER® device clinical study.
“The close collaboration between medical community and manufacturer has allowed for numerous innovations and improvements along the way, providing for more accurate deployment and better outcomes.”
 “As EVAR became more prevalent and additional patient needs were uncovered, it was important that W. L. Gore & Associates—the global materials science company behind the device—continue exploring and improving EVAR solutions,” said Davison, Abdominal Aortic Global Business leader at Gore.
“As EVAR became more prevalent and additional patient needs were uncovered, it was important that W. L. Gore & Associates—the global materials science company behind the device—continue exploring and improving EVAR solutions,” said Davison, Abdominal Aortic Global Business leader at Gore.
“We recognized the broader potential of the device but knew that there was still a need to keep improving, keep innovating, to help address even more patients’ unique needs and anatomies.”
Over the next two decades, Gore developed and studied additional solutions within its EVAR portfolio, receiving approvals for larger trunk and contralateral limb diameters, an iliac branch device, and a next generation of the EXCLUDER® device that is conformable and offers optional angulation control—allowing physicians more treatment options to consider for their patient’s anatomy.

Davison said that at Gore, collaboration with the medical community is key to developing solutions that continue to advance patient care. “We are immensely grateful to the physicians who have put their trust in our devices to help their patients maintain their quality of life and those who have partnered with us as we continue exploring future solutions for AAA patients,” he said.
*Based on the number of trunk-ipsilateral legs distributed.
Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. Please see accompanying prescribing information in this journal. Products listed may not be available in all markets. GORE, Together, improving life, EXCLUDER and designs are trademarks of W. L. Gore & Associates. ©2022 W. L. Gore & Associates, Inc.












