“A strategy of adding rivaroxaban 2.5mg twice daily to aspirin should be considered after lower extremity bypass regardless of conduit type,” concluded Nicholas Govsyeyev, MD, during the William J. von Liebig Forum at the Vascular Annual Meeting (VAM) in San Diego. Govsyeyev was giving an update on the VOYAGER PAD trial, addressing the efficacy of rivaroxaban and aspirin in peripheral arterial disease (PAD) patients with venous and prosthetic surgical bypass conduits.
“The optimal antithrombotic therapy following infrainguinal bypass is not known,” Govsyeyev began. He noted that two major trials have examined the role of dual antiplatelet therapy (DAPT) and oral anticoagulation following infrainguinal bypass—CASPAR and DUTCH BOA. While both these trials had neutral results, the presenter detailed, there was significant heterogeneity by conduit type, with the CASPAR trial showing the potential benefit of DAPT in prosthetic conduits and the DUTCH BOA trial demonstrating the potential benefit of anticoagulation in venous conduits. Both trials, however, had a significant increase in major bleeding.
“The VOYAGER PAD trial provides a large, prospective database to further examine the optimal antithrombotic regimen following infrainguinal bypass,” Govsyeyev remarked. This trial randomized 6,554 patients with symptomatic PAD undergoing lower extremity revascularization and receiving standard of care to rivaroxaban 2.5mg taken twice daily or placebo, with standard of care consisting of aspirin, clopidogrel use per the investigator’s discretion, and the encouragement of statin use.
Govsyeyev summarized the key findings: “The trial demonstrated a 15% reduction in the primary composite endpoint of acute limb ischemia, major amputation, myocardial infarction, ischemic stroke, and cardiovascular death; bleeding was increased, but overall incidence was low with rivaroxaban; there was no significant interaction for efficacy or safety on the basis or surgical or endovascular revascularization; and surgical patients’ risk for primary endpoint events was reduced by 19% with rivaroxaban.”
The objectives of the current study, Govsyeyev explained, were to compare limb outcomes with venous versus prosthetic conduits in VOYAGER PAD patients who underwent bypass, and to evaluate whether the efficacy of rivaroxaban is consistent across conduit types.
In order to assess this goal, the researchers used a multivariate adjusted model to determine the risk of limb outcomes in patients with venous and prosthetic conduits, and Cox proportional hazards to assess for efficacy.
Govsyeyev detailed that a total of 1,448 (66%) patients underwent bypass in the VOYAGER trial. Breaking this down by conduit type, he noted that prosthetic use was slightly more frequent (773 patients; 54%) than venous use (646 patients; 46%).
In terms of baseline characteristics, the speaker communicated that the median age of the venous cohort was 65 (59–70) compared to 66 (61–72) in the prosthetic group, and that the number of female patients in the venous group was slightly lower than in the prosthetic group, at 17 and 22, respectively. Medical comorbidities were not different between the two groups, he added.
With regard to distribution by target artery, Govsyeyev stated that venous conduits were more likely to be used below the knee, whereas three-quarters of prosthetic conduits had an above-the-knee target artery.
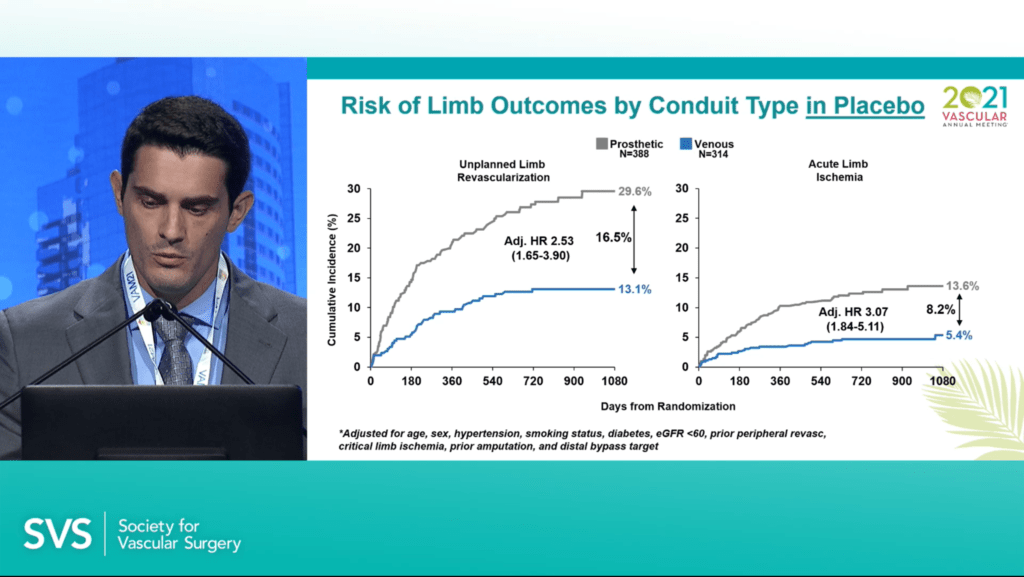
Govsyeyev reported that, in the placebo group, the cumulative incidence of unplanned limb revascularization at three years from randomization was 29.6% in the prosthetic cohort (n=388) compared to 13.1% in the venous cohort (n=314), with a risk difference of 16.5%. Regarding acute limb ischemia, the figures were 13.6% and 5.4%, respectively, with an 8.2% risk difference.
The presenter then outlined results related to the efficacy of rivaroxaban on the primary composite endpoint by conduit type. “Prosthetic patients were at higher risk for primary endpoint events, and this was reduced with rivaroxaban, with an absolute risk difference of 6.4%,” Govsyeyev revealed. Among the venous conduits, the data showed an absolute risk difference of 7.9% with rivaroxaban. Govsyeyev added that there was no significant interaction by conduit type and overall bleeding was quite low in the rivaroxaban and placebo groups.
Furthermore, the speaker informed the audience that there was consistent benefit of rivaroxaban on limb outcomes regardless of conduit type. He summarized the results: “Prosthetic conduits had a higher risk for limb outcomes; acute limb ischemia was reduced in both venous and prosthetic conduits with rivaroxaban, with a particularly strong benefit among venous conduits with a greater than 50% reduction; major amputation was reduced in venous conduits but not in prosthetic; unplanned limb revascularization was reduced in prosthetic but not in venous; and there was no significant interaction for any limb outcome by conduit type.”
Addressing the VAM audience, Govsyeyev concluded that a strategy of rivaroxaban 2.5mg twice daily reduces irreversible harm events and increases bleeding with a 6:1 benefit risk ratio, and that the magnitude of benefit appears robust in surgical patients. He added that prosthetic conduit patients are at a roughly three-fold risk of acute limb ischemia and unplanned revascularization relative to those receiving venous conduits, and that the benefit of rivaroxaban is consistent across conduit types with around a 2% absolute reduction in acute limb ischemia and around a 6% reduction in primary outcome. Taking all these factors into account, Govsyeyev commented: “A strategy of adding rivaroxaban 2.5mg twice daily to aspirin should be considered after lower extremity bypass regardless of conduit type.”