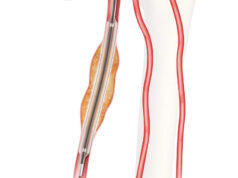
Hancock Jaffe Laboratories recently revealed that the United States Patent and Trademark Office (USPTO) issued the first patent covering the company’s VenoValve. The patent is entitled Implantable Vein Frame and is U.S. patent number 10,959,841.
The company recently announced that only 28 days after filing, the Food and Drug Administration (FDA) approved an Investigational Device Exemption (IDE) application to begin the U.S. pivotal trial for the VenoValve. Known as the SAVVE study, the trial is a prospective, non-blinded, single-arm, multicenter study of 75 chronic venous insufficiency (CVI) patients to be enrolled at up to 20 U.S. centers.
“We will continue to work with agencies like the FDA and USPTO, and other regulatory authorities throughout the world towards our ultimate goal of reaching the millions of patients suffering from CVI”, said Hancock Jaffe CEO, Robert Berman. “We believe that the VenoValve will set a new standard of care for CVI patients and will establish Hancock Jaffe as a leading provider of innovative medical devices for peripheral vascular disease”.
Leading hospitals and top vascular surgeons throughout the U.S. have expressed interest in participating in the SAVVE study. The company has begun the process of fulfilling conditions for study initiation outlined by the FDA and seeking Institutional Review Board (IRB) and other necessary approvals from potential SAVVE sites. The company expects to begin patient enrollment in the third quarter of 2021 and will provide periodic updates.