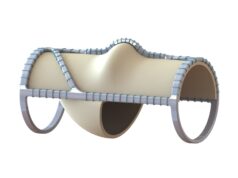
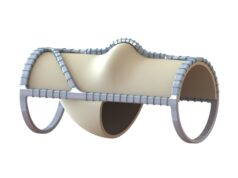
The Food and Drug Administration (FDA) has issued a letter to Envveno Medical stating that its VenoValve technology is “not-approvable,” a company press release published today reports. The letter was issued in response to Envveno’s premarket approval (PMA) application for VenoValve—a surgical replacement venous valve for treating severe deep chronic venous insufficiency (CVI).
According to Envveno, the letter indicates that the FDA completed its review of the VenoValve PMA application and determined that it is unable to approve the PMA for the VenoValve in its current form. “In particular, the FDA indicated that the favorable revised Venous Clinical Severity Score (rVCSS) data generated by the [SAVVE] study to show clinical improvement, together with the improvements in pain scores and venous-specific quality-of-life indicators was not sufficient on its own to determine favorability of the benefit risk profile for the VenoValve,” the company press release reads. “Without a specific hemodynamic measurement that correlates with patient improvement, the FDA raised concerns about bias and the possibility that clinical improvement occurred as a result of the patients being enrolled in a study.”
The FDA also focused on safety concerns which were attributed to the VenoValve open surgical procedure, and that required rehospitalizations. Envveno notes that it “would not expect to see similar safety events with a non-surgical replacement valve”.
“We are obviously disappointed by the FDA’s decision. The results showed that a high percentage of the patients in the SAVVE study, who all previously failed standard-of-care treatments, showed significant clinical improvement after receiving the VenoValve. With the VenoValve being the only difference in their care, it is hard to not attribute the improvement to the VenoValve,” said Robert Berman, Envveno’s chief executive officer, in the press release. “We remain committed to the 2.5 to 3.5 million patients suffering from severe deep venous CVI in the US and who have no effective treatment options and will continue to work with the FDA on new criteria to demonstrate the safety and effectiveness of our devices.”
Envveno advises that it is reviewing the feedback from the FDA and is assessing all options, which may include a meeting to discuss requirements for a potential resubmission of the VenoValve or appeal of the decision along with appropriate next steps. The company adds that it also expects to apply the “key learnings” from this FDA approval process as it advances Envve, its non-surgical replacement venous valve for which it is preparing an investigation device exemption (IDE) application.