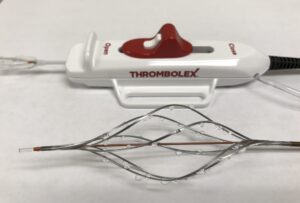
Thrombolex and Aidoc have announced a strategic partnership aimed at revolutionising the treatment of acute pulmonary embolism (PE), a press release reports.
This strategic collaboration, the release details, will leverage Aidoc’s artificial intelligence (AI) technology to accelerate patient identification and enrolment for Thrombolex’s single-session RAPID-PE study—a prospective, single arm, multicentre, postmarket clinical registry evaluating the efficacy and safety of the Bashir endovascular catheters in the treatment of acute intermediate-risk PE.
Thrombolex’s Bashir endovascular catheters are engineered to immediately restore blood flow, rapidly resolve blood clots and help reduce complications. Aidoc’s AI capabilities and tailored mobile application will provide local healthcare teams at RAPID-PE trial sites with timely alerts on patients who meet the inclusion criteria.
“Integrating Aidoc’s advanced imaging solutions with our innovative technology will help to improve the diagnosis and treatment process for acute PE,” said Michael Cerminaro, president and chief executive officer of Thrombolex. “This partnership demonstrates our commitment to leveraging cutting-edge technology to enhance patient outcomes, streamline clinical workflows and expedite enrolment in our RAPID-PE study.”
Aidoc’s proprietary technology features dedicated US Food and Drug Administration (FDA)-cleared algorithms for PE, central PE, incidental PE (iPE), and RV/LV ratio. It also alerts care teams to both expected and unexpected PE cases for faster triage and coordination with the right specialists. Recent studies showed the mean hospital length of stay decreased by 36.7% and the annual volume of patients referred for interventional therapies rose by 68% compared to the period before the implementation of Aidoc PE AI.
Wissam Jaber (Emory University, Atlanta, USA), co-national physician investigator for RAPID-PE, said: “Combining Thrombolex’s innovative PML [pharmacomechanical] technology with Aidoc’s advanced AI-powered imaging solutions enhances speed to diagnosis and treatment efficiency for acute PE patients. This collaboration, along with RAPID-PE’s groundbreaking single session investigational protocol, represents a major step forward in improving patient outcomes and advancing the standard of care in our field.”
The RAPID-PE study will utilise a therapy protocol designed as a single session treatment (less than one hour), with no need for an intensive care unit (ICU) stay and a reduced dose of lytics (only 4mg per pulmonary artery).
“We are proud to collaborate with Thrombolex on this groundbreaking clinical study by leveraging our advanced AI technology,” stated Tom Valent, chief business officer, Aidoc. “Thrombolex’s leadership and innovative solutions are setting new standards in patient care, and our collaboration is a testament to the power of combining cutting-edge medical devices with AI-powered solutions for the joint mission of improving patient care.”
The RAPID-PE clinical study is currently qualifying multiple centers across the USA.