Thirty-day results from the PERFORMANCE III study of integrated embolic protection (IEP) in transcarotid artery revascularization (TCAR) demonstrate “very low” 30-day event rates. Sean Lyden, MD, chair of vascular surgery at the Cleveland Clinic in Cleveland, Ohio, shared this finding during a late-breaking trials session at the 2025 Vascular Interventional Advances (VIVA) conference (Nov. 2–5) in Las Vegas.
The prospective, multicenter PERFORMANCE III study evaluated the safety and effectiveness of the Neuroguard IEP system (Contego Medical), 70cm carotid stent and the Neuroguard IEP embolic protection system for direct TCAR in 146 patients across 26 clinical sites. The Neuroguard IEP embolic protection system includes both a direct access kit and a blood flow reversal system.
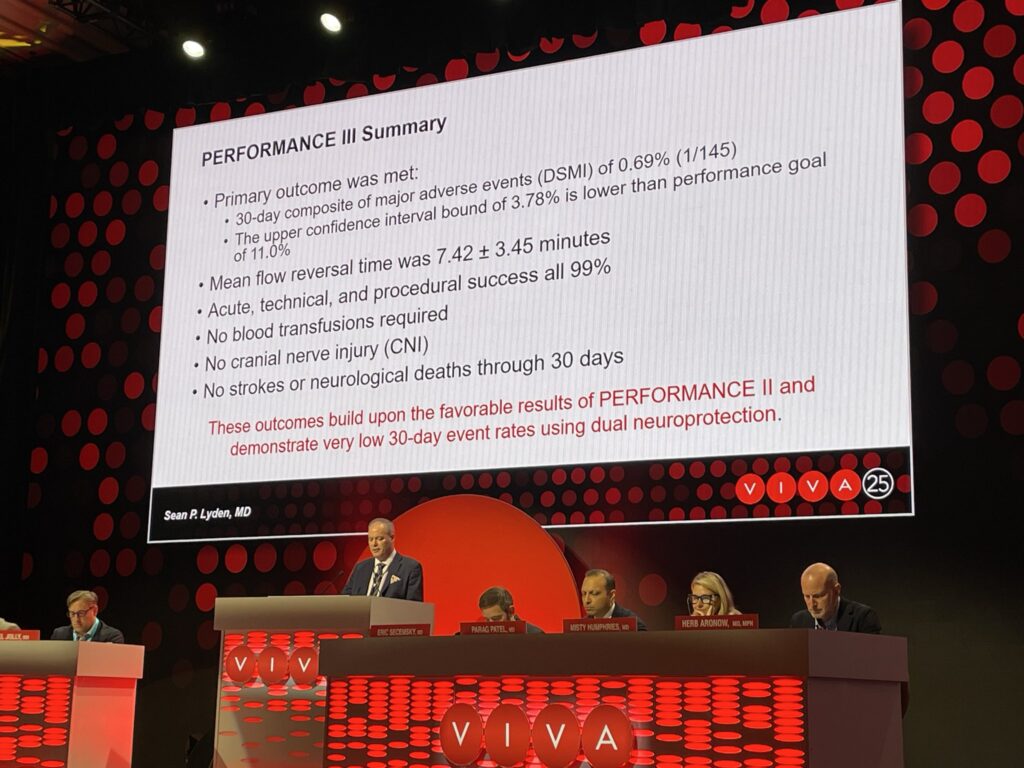
“The 30-day event rates in the PERFORMANCE III study are the lowest reported in a prospective, multicenter carotid artery revascularization study,” said Lyden, who is co-national principal investigator of PERFORMANCE III.
In the PERFORMANCE III study, the 30-day stroke rate was 0% per the intention-to-treat analysis, with zero myocardial infarctions, zero cranial nerve injuries, zero neurological deaths, and zero stent thromboses reported. The primary endpoint of major adverse events through 30 days was 0.69% (intention-to-treat) and 0% (per-protocol), which Lyden noted was significantly lower than the predefined study performance goal.