Endologix recently announced completing the acquisition of PQ Bypass, a medical technology company pioneering a first-of-its-kind technology that addresses severe peripheral arterial disease (PAD).
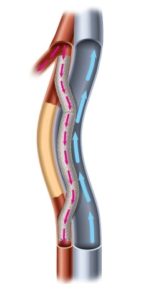
PQ Bypass’ proprietary Detour platform for percutaneous femoropopliteal bypass has been designated by the Food and Drug Administration (FDA) as a Breakthrough Device. The Detour system consists of the TORUS stent graft and the PQ crossing device. The Detour system is currently being studied in a U.S. and European clinical trial, DETOUR2.
“The acquisition of PQ Bypass is a seminal moment in Endologix’s history, building upon our leadership in the treatment of abdominal aortic aneurysm to champion disruptive technologies for the treatment of vascular disease,” said Richard Mott, CEO and chairman of Endologix.
“We intend to actively pursue new and innovative vascular technologies that are clinically relevant to surgeons, hospitals, and patients, with a commitment to world-class medical education, clinical research, and excellent procedural outcomes.”












