Given the absence of a safety signal in data from the VOYAGER PAD trial, a new analysis examined the potential benefit of drug-coated device versus non-drug-coated device treatment for reducing limb outcomes.
Connie Hess, MD, an associate professor of cardiology at the University of Colorado in Aurora, presented the most recent findings in a late-breaking data session at this year’s Vascular Interventional Advances conference (VIVA 2020) held Nov. 6–8 virtually, reporting no excess mortality and improved limb outcomes with drug-coated device use in peripheral arterial disease (PAD).
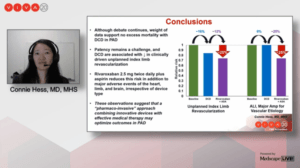
Hess noted that patency remains a challenge, and that drug-coated devices are associated with a reduction in clinically-driven unplanned index limb revascularization.
However, she relayed that rivaroxaban 2.5mg twice daily plus aspirin reduces this risk, in addition to reducing major adverse events of the heart, limb, and brain, irrespective of device type. “These observations suggest that a ‘pharmaco-invasive’ approach combining innovative devices with effective medical therapy may optimize outcomes in PAD,” she told the VIVA audience.
The co-primary outcomes for this analysis were unplanned index limb revascularization and major adverse limb events, defined as acute limb ischemia or major amputation of vascular cause. As drug-coated use was not randomized, inverse probability treatment weighting was used to account for known confounders.
Hess detailed that use of a drug-coated device was associated with a significant 16% reduction in relative risk of clinically-driven unplanned index limb revascularization (hazard ratio [HR] 0.84, 95% confidence interval [CI] 0.76–0.92) but was not associated with a reduction in MALE (HR 1.08, 95% CI 0.9–1.3).











