 The interim results of a first-in-human trial of a sirolimus-eluting covered stent graft—Solaris DE (Solaris Endovascular)—for the treatment of dialysis access dysfunction demonstrate the promise of the device in treating vascular access outflow stenosis, an investigator in the trial has commented.
The interim results of a first-in-human trial of a sirolimus-eluting covered stent graft—Solaris DE (Solaris Endovascular)—for the treatment of dialysis access dysfunction demonstrate the promise of the device in treating vascular access outflow stenosis, an investigator in the trial has commented.
Results of the DEScover trial, evaluating the safety and efficacy of the Solaris drug-eluting endovascular stent graft in patients with arteriovenous fistulae (AVF) and arteriovenous grafts (AVG), were presented at the 2025 Transcatheter Cardiovascular Therapeutics (TCT) meeting in San Francisco (Oct. 25–28), and again at the 2025 VEITHsymposium in New York City (Nov. 18–22), by Leonardo Harduin, MD, a vascular surgeon at State University of Rio de Janeiro in Rio de Janeiro, Brazil, who described the device as opening a “new avenue” in dialysis access treatment.
Balloon angioplasty is the current standard of care for the treatment of vascular access stenosis, but recurrent stenosis up to one year after treatment remains high among these patients, Harduin described in his presentation. Stent grafts have been considered as one option to address this issue, he noted, though such technologies have been held back by unfavorable rates of target lesion primary patency (TLPP).
“The reason for stent graft failure is neointernal hyperplasia that occurs mainly on the edge of the device and sometimes in the middle of the stent graft,” Harduin detailed. The Solaris DE covered stent is built with an impermeable electrospinning PTFE membrane to limit cellular migration, allied with the sirolimus coating to block neointimal hyperplasia and restenosis. “Sirolimus is a safe and effective cytostatic drug that has been used for a long time in interventional cardiology in coronary arteries to prevent neointimal hyperplasia, restenosis, and thrombosis,” said Harduin.
Animal studies of Solaris DE have, to date, shown the persistence of the sirolimus on the outer edge of the device, he said, with one study comparing it to a conventional stent graft finding no instances of neointimal hyperplasia at 60 days following Solaris DE implantation, compared to 4.9mm3 with the conventional device.
Investigators therefore established the DEScover trial, a phase II, first-in-human, feasibility trial, to evaluate the safety and effectiveness of the Solaris DE in the venous outflow compared to percutaneous transluminal angioplasty (PTA) for treatment of de novo or restenotic lesions of native AVF and for graft-vein stenosis of AVG. Patients were eligible for inclusion if they had AVF/AVG with clinical function and a hemodynamically-significant restenosis in the outflow circuit.
Patients in the AVF cohort were randomized 1:1 after successful predilatation with a high-pressure balloon to receive either Solaris DE or PTA. The AVG cohort was a single-arm group, with patients only receiving the covered stent. The primary endpoints were targeted lesion primary patency (TLPP) rates at six months and freedom from any serious adverse events at 30 days.
Harduin reported that 65 patients completed the six-month follow-up, with 19 patients in the AVG group, 22 patients in the AVF group who received Solaris DE, and 24 patients in the AVF group who underwent standard PTA treatment. Hypertension was less frequent in the PTA group, and brachiocephalic fistulas with cephalic arch stenosis and de novo lesions were more similarly proportioned in both groups.
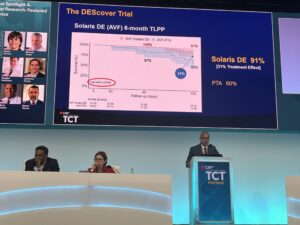
Regarding the results, Harduin detailed that there was 100% freedom from adverse events affecting the access or venous outflow circuit in all groups at 30 days after the index procedure. Reporting an overall TLPP rate of 95% at six months across both groups, Harduin detailed that there was 100% TLPP at six months for patients receiving Solaris DE in the AVG cohort, with a TLPP rate of 91% at six months for AVF patients who received the device. This compared to a 60% rate of TLPP for patients who underwent PTA treatment.
“These interim analyses confirm the safety of Solaris drug-eluting covered stent in humans,” Harduin said of the results in the concluding remarks of his presentation at TCT 2025. “These early results suggest it may open a new avenue in dialysis access treatment.”
Enrollment is expected to be finalized in the coming weeks and the complete six-month results will be presented in the second half of 2026.












