A rupture prediction-based algorithm is set to enhance patient selection for abdominal aortic aneurysm (AAA) repair. This is according to Randy Moore, MD, a vascular surgeon at the University of Calgary in Calgary, Canada, who, at the 2024 VEITHsymposium (Nov. 19–23) in New York City reported that the ViTAA system (ViTAA Medical Solutions) can predict rupture risk independent of aneurysm size and post-endovascular aneurysm repair (EVAR) neck behavior.
Before sharing the results of a retrospective analysis of computed tomography (CT) factors determining AAA wall weakness and strength in AAAs, Moore—who is chief medical officer for ViTAA Medical—began his presentation by outlining an unsuccessful case that highlighted for him an unmet need in vascular surgery. Showing a CT scan, the presenter recalled: “Fifteen years ago, this patient died on my operating table. He wasn’t supposed to die; his aneurysm was small, and I was following guidelines.”
Moore noted that cases such as this are not unique. “In fact,” he said, “a number of large database studies with thousands of patients have demonstrated that up to 12% of patients may have a rupture below size threshold.” Despite this, Moore highlighted that there are investigators who are suggesting the size thresholds for repair can safely be increased due to a low overall risk of rupture.
Moore’s explanation for this “confusion” is the “absolute reliance on AAA size to risk stratify.” In his VEITHsymposium presentation, it was Moore’s aim to suggest to the audience that this firm trust in aneurysm size “remains fundamentally flawed.” “We really need to analyze the aortic wall,” he said. “That’s the key.”
Other investigators have tried to analyze the aortic wall using traditional finite element analyses and stress-based indices, Moore shared, before noting that several publications in the engineering space have underscored the deficits of such strategies. “You’re not taking into account the actual intrinsic patient wall characteristics,” the presenter stated. “In other words, the same stress or force applied to two aortas is going to give you two different clinical outcomes based on the tissue characteristics.”
“We took a different tack,” Moore told the VEITHsymposium audience. Over the past decade, the presenter recounted that he and a team of engineers have processed over 200 aortic specimens. The aim has been “to develop technology that precisely takes into account the integral strength of the aortic wall,” Moore shared.
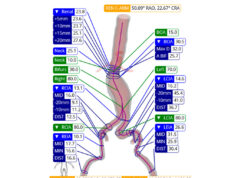
The presenter explained that, based on a cardiac-gated scan that allows a clinician to track the motion of the aortic wall, data are uploaded to the cloud where a combination of algorithms assess the intraluminal thrombus thickness, time-averaged wall shear stress with computational fluid dynamics and a peak strain module, all of which are then combined as an output called a regional aortic weakness (RAW) map.
“These maps allow you for the first time to virtually assess the mechanical properties of the aortic wall, not based on population data but based on the individual patient sitting in front of you in the clinic,” Moore elaborated. “We now have an AAA wall analysis that includes wall tissue strength characteristics irrespective of size.”
On use of the technology so far, Moore reported that it has played a role in analyzing landing zones for EVAR and has demonstrated that implanting a stent into a weak infrarenal aortic neck is more likely to result in a type 1 endoleak. “That’s not a surprise,” Moore commented, “but now we can actually measure that.”
The technology can also be used for surveillance, Moore continued. He explained that this is due to its ability to analyse peak strain post-EVAR and to point out “which endoleaks actually need to be treated, and which can be safely ignored.”
Furthermore, Moore shared that the technology is being used with artificial intelligence (AI) algorithms to predict rapid growth, which he describes as a “surrogate marker for negative outcomes.” These data were shared at the 2023 VEITHsymposium.
The “holy grail,” however, is rupture prediction. Moore detailed that he and his team previously published on the ability to identify the site of aortic rupture, but that the more recent aim had been to complete a study looking at the risk of rupture and the ability to predict rupture using the ViTAA technology. “This was a very difficult study to complete,” the presenter remarked, citing specifically the challenge of finding patients with images prior to rupture.
Moore detailed that he and colleagues retrospectively reviewed 38 patients with rupture and matched them in terms of size, age and demographics to 38 non-ruptured patients before conducting a ViTAA analysis. “We wanted to make sure we included patients who had a range of aneurysm sizes to reflect real-world practice—roughly 4.6cm all the way up to 12cm,” he said.
The team then completed a traditional analysis using finite element and stress-based indices, finding no statistical difference between ruptured and non-ruptured patients. With the ViTAA analysis, however, Moore reported that the team was able to identify a “highly predictive” tool and one that “provides the clinician with an at-a-glance interpretation of aortic wall risk with an accuracy of 92%, a sensitivity for rupture prediction of 100%, specificity of 83% and an area under the curve of 86%.”
“Our AAA wall analysis based on tissue strength and strain allows for patient-specific wall maps that will eventually allow us to more precisely inform patient care,” Moore concluded. “We have a rupture prediction algorithm that will enhance patient selection for repair so that patients like mine 15 years ago will no longer die unnecessarily.”
In Moore’s closing statement, he outlined the longer-term goal of “no longer seeing exclusive reliance on aortic size to determine patient care.”
Data collection ‘absolutely crucial’
In discussion time following Moore’s presentation, Rao Vallabhaneni, MD, a professor of vascular surgery at theUniversity of Liverpool in Liverpool, England, commented on the potential limitations of the 38-patient sample size of the study.
“The difficulty is the great heterogeneity within one person’s aneurysm from different areas of the same aneurysm and between patients,” Vallabhaneni began. “It is such highly heterogenous tissue that I don’t think your sampling is enough to develop a reliable tool.”
While acknowledging Vallabhaneni’s concern, Moore stressed that “the proof is in the pudding”. “With a limited number of 38 ruptured patients, we showed a sensitivity of 100% in terms of rupture prediction,” he reiterated.
However, Moore closed his reply by agreeing with Vallabhaneni that data accumulation is “absolutely critical,” going on to share that he and his team have initiated a series of prospective registries they hope will answer further questions about the technology.