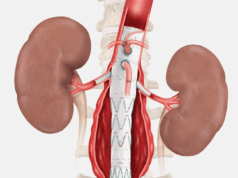
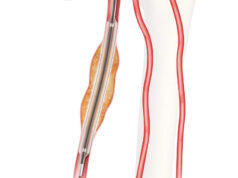
Getinge recently announced that it has received Food and Drug Administration (FDA) premarket approval (PMA) for the iCast covered stent system. The device is now available as a bridging stent for the treatment of patients with aneurysmal disease.
Getinge states that this new indication enables physicians to preserve blood flow between a branch vessel and an endovascular graft that is approved for use with the iCast covered stent.
In March 2024, Getinge and Cook Medical entered into a distribution agreement under which Cook assumed sales, marketing, and distribution responsibilities for the iCast covered stent system in the U.S. The product, now with its expanded bridging stent indication, will continue to be distributed by Cook Medical.