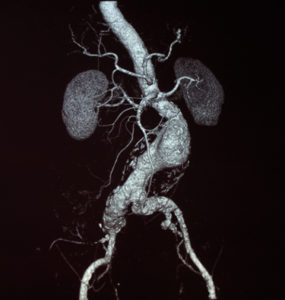
The first U.S. patient has been treated as part of the SOCRATES trial (Short neck AAA randomized trial—ESAR and FEVAR), which compares the safety and performance of endosuture aneurysm repair (ESAR) with fenestrated endovascular aneurysm repair (FEVAR) for the treatment of abdominal aortic aneurysms (AAAs) with a short neck.
The maiden procedure performed Stateside was carried out by Brant Ullery, MD, the medical director of vascular and endovascular surgery at Providence Heart and Vascular Institute in Portland, Oregon, who is a SOCRATES trial co-principal investigator.
The randomized, postmarket, head-to-head study was designed to determine whether clinical outcomes of ESAR and FEVAR are equivalent in the treatment of infrarenal AAAs with a core lab-measured short proximal neck length of 4–15mm and minimal infrarenal sealing zone of 8mm, the trial’s sponsor, the Foundation for Cardiovascular Research and Education (FCRE), states.
Organized globally by FCRE in collaboration with Medtronic as funding partner, it randomizes patients 1:1 to either ESAR with the Endurant II/IIs stent graft system (Medtronic) and Heli-FX EndoAnchor system (Medtronic), or FEVAR with the Zenith Fenestrated AAA endovascular graft (Cook Medical) or Anaconda fenestrated stent graft (Terumo).
SOCRATES is slated to enroll approximately 204 patients at up to 40 sites globally. The prespecified safety endpoint is freedom from major adverse events through 30 days. The composite effectiveness endpoint is technical success at index procedure, freedom from type IA or type III endoleaks, freedom from aneurysm-related mortality, and freedom from secondary reinterventions through 12 months.
Heli-FX has FDA clearance for distribution in the U.S. and CE Mark approval for distribution in Europe. Zenith Fenestrated is available in the U.S. and Europe, and Anaconda is available outside the U.S.
Speaking on the design of SOCRATES at this year’s Leipzig Interventional Course (LINC 2023) in Leipzig, Germany, in June, Ullery’s co-principal investigator Giovanni Torsello, MD, from St Franziskus Hospital in Münster, Germany, expanded on the intentions of the trial, which he stated is the “first comparative” study to compare ESAR and FEVAR.
Torsello told LINC attendees how hostile aortic neck can lead to “loss of proximal seal over time,” noting how short necks are associated with increased risk of type IA endoleak and secondary procedures, ultimately asking: “How should we treat our patients with a short neck?”
Which is where SOCRATES seeks to step in.
“If we can build the first comparative trial in the treatment of such patients, and we can learn more about the fate of those treated in these ways, it will be a fantastic trial,” Torsello said.
The extension of trial enrollment to the United States from its initial European starting place would “make a great contribution to this prospective study,” he added.












