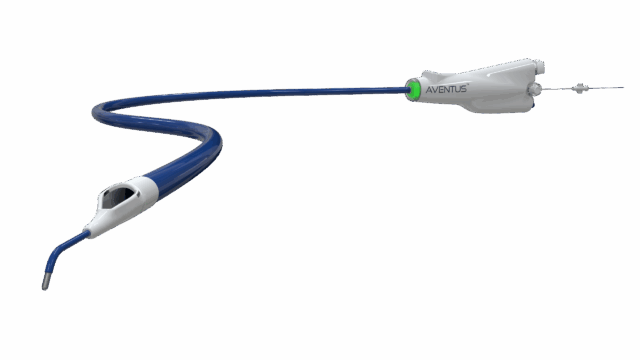
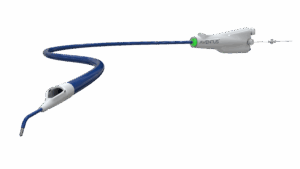
 Inquis Medical has announced that its Aventus thrombectomy system has received 510(k) clearance from the US Food and Drug Administration (FDA) for an expanded indication to treat pulmonary embolism (PE).
Inquis Medical has announced that its Aventus thrombectomy system has received 510(k) clearance from the US Food and Drug Administration (FDA) for an expanded indication to treat pulmonary embolism (PE).
The Aventus System is a next-generation mechanical thrombectomy platform developed in close collaboration with physicians to address critical limitations of current technologies.
The Aventus thrombectomy system was previously cleared by the FDA for use in the peripheral vasculature. Additionally, the Aventus clot management system received FDA clearance for use with the Aventus thrombectomy system to enable autologous blood transfusion, allowing reinfusion of filtered aspirated blood and supporting efficient, blood-conserving clot removal. This most recent clearance extends the platform’s indication to include the treatment of pulmonary embolism.
This regulatory milestone follows the successful completion of the AVENTUS pivotal trial, the first U.S. investigational device exemption (IDE) study to evaluate aspiration thrombectomy with filtered blood reinfusion in intermediate-risk PE patients.
The trial demonstrated “excellent” safety and performance across a broad range of clinical settings, with no device-related major adverse events and rapid improvement in right heart strain, according to the company. The results were presented as a late-breaking clinical trial at the 2025 Society of Cardiovascular Angiography and Interventions (SCAI) scientific sessions (May 1–3) in Washington, D.C., and were simultaneously published in JSCAI.