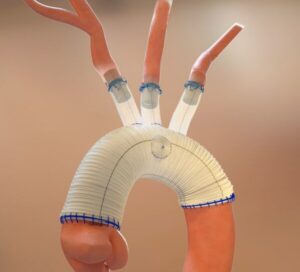
The Food and Drug Administration (FDA) has approved the expansion of Aquedeon Medical’s investigational device exemption (IDE) clinical trial evaluating the Duett vascular graft system, a company press release reveals. Duett is a novel device designed to facilitate open surgical repair of aortic arch aneurysms and dissections, including hybrid procedures.
Aquedeon Medical notes that it is developing the Duett system “to address a critical need in open surgical thoracic aortic arch reconstruction, a complex and high-risk procedure typically performed during deep hypothermic circulatory arrest (DHCA) while the patient is on cardiopulmonary bypass”.
The Duett system, Aquedeon Medical shares, is engineered to “simplify and accelerate” the vascular anastomosis process, with the goal of reducing DHCA duration and re-establishing cerebral perfusion more rapidly.
Aquedeon Medical launched its two-stage IDE clinical trial in 2024 to evaluate the safety and effectiveness of the Duett system. With the recent FDA approval, the study will now expand to enroll up to 90 patients across additional clinical sites in the U.S.