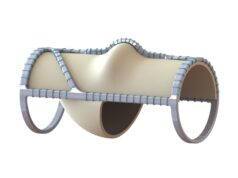
Hancock Jaffe Laboratories today (Wednesday, Aug. 4) announced that the Food and Drug Administration (FDA) has granted Breakthrough Device Designation status to the company’s VenoValve device, a potential treatment option for chronic venous insufficiency (CVI).
“We are very pleased to have the opportunity to work with the FDA on an expedited basis as we try to bring relief to the millions of patients who suffer from deep venous CVI and who currently have no effective treatment options,” said Hancock Jaffe CEO Robert Berman. “The VenoValve significantly improved the lives of the patients in our first-in-human study, and we hope to replicate that success in our SAVVE U.S. clinical trial.”
Data from the VenoValve first-in-human study, presented in December 2020, indicated that average patient improvement in reflux was 54%, average improvement in disease manifestations was 56%, and average improvement in pain was 76%, all at one-year post-VenoValve surgery compared to pre-surgery levels, Hancock Jaffe reported in a press release. In addition, there were no material adverse events (MAEs) at 30 days post-VenoValve implantation.
The primary endpoints for the Company’s SAVVE (Surgical anti-reflux venous valve endoprosthesis) U.S. pivotal trial will be the same as for the first-in-human trial. The primary safety endpoint is the occurrence of MAE in less than 10% of patients at 30 days post-VenoValve implantation, and the primary effectiveness endpoint is improvement of reflux equal to or greater than 30% at six months following VenoValve surgery. MAEs are defined as the composite of all-cause mortality, deep wound infection, major bleeding, ipsilateral deep vein thrombosis (DVT) or pulmonary embolism. Improvement of Venous Clinical Severity Score (VCSS) and visual analog scale (VAS) scores are also included in the SAVVE study as secondary endpoints.
Preparation to begin enrollment of 75 patients at up to 20 centers throughout the U.S. for the SAVVE pivotal trial is being finalized, with the first patient expected to be enrolled in the study within the next 60 days, Hancock Jaffe added.