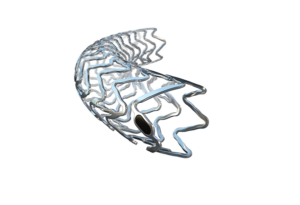
Biotronik has been granted Breakthrough Device designation (BDD) from the US Food and Drug Administration (FDA) for the Freesolve below-the-knee (BTK) resorbable magnesium scaffold (RMS).
The Freesolve BTK RMS is designed for individuals suffering from chronic limb-threatening ischaemia (CLTI).
To qualify for a Breakthrough Device designation, a device technology must address an unmet need and show that it has the potential to provide for more effective treatment of life-threatening diseases or irreversibly debilitating conditions. The goal of the programme is to provide patients and clinicians with timely access to these breakthrough treatments by accelerating their development, assessment and review while maintaining regulatory standards for pre-market approval.
A press release notes that the newly CE-launched Freesolve RMS for coronary artery lesions, based on the Biomag magnesium alloy and the proven Orsiro drug-eluting stent (DES) coating technology, provides safety, improved deliverability, and optimal performance and vessel support during and after implantation. It has shown 99.6% degradation of magnesium observed 12 months after implantation in coronary arteries, the release details.
These characteristics of the Freesolve RMS may be of particular value in BTK interventions, where vessel scaffolding is desired in the short-term to resist vessel recoil, yet ultimately leave the vessel implant-free.
“Biotronik’s focus on vascular interventional excellence is evident in our strategic investments and persistent dedication to innovation”, said Jörg Pochert, president of Vascular Intervention at Biotronik. “Our efforts to expand therapeutic possibilities, underlined by the introduction of the Freesolve RMS for coronary artery disease treatment, will continue in the BTK indication with this groundbreaking innovation.”
“This Breakthrough Device designation for the Freesolve RMS for BTK treatment is a significant milestone in advancing treatment options. Biotronik is committed to design our products to enhance the lives of patients,” stated Ryan Walters, US president at Biotronik. “Our next generation RMS represents a leap forward over existing resorbable technology, incorporating technical innovations intended to address physicians’ needs and optimise outcomes for patients suffering from CLTI.”