Tag: Surmodics
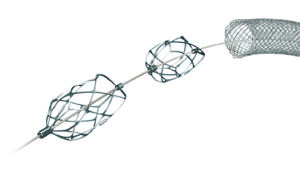
Surmodics announces commercial release of Pounce XL thrombectomy system
Surmodics has announced the commercial release of the Pounce XL thrombectomy system for endovascular removal of acute or chronic clot from peripheral arteries.
Intended for removal...
Surmodics announces early results from PROWL registry of Pounce thrombectomy system
Surmodics has announced that early results of a subset of 60 real-world acute, subacute, and chronic limb ischaemia patients from its PROWL registry study...
Pounce Thrombectomy Platform use in acute limb ischemia: Vascular surgeons weigh...
This advertorial is sponsored by Surmodics.
Interventions for acute limb ischemia (ALI) represent up to 16% of the case volume for vascular surgeons and cost...
Surmodics receives FDA 510(k) clearance for Pounce XL thrombectomy system
Surmodics has announced that it has received Food and Drug Administration (FDA) 510(k) clearance for its Pounce XL thrombectomy system.
The Pounce system is indicated...
TRANSCEND 36-month data “continue to demonstrate safe and effective performance” of...
Peter Schneider (University of California San Francisco, San Francisco, USA) recently presented 36-month data from Surmodics' TRANSCEND clinical trial at the VEITHsymposium 2023 (14–18...
The top 10 most popular Vascular Specialist stories of July 2023
In the previous month, Vascular Specialist delivered a range of leading commentary from the Society for Vascular Surgery (SVS) in response to the New...
Surmodics receives FDA approval for the SurVeil drug-coated balloon
Surmodics has announced the receipt of Food and Drug Administration (FDA) approval for the SurVeil drug-coated balloon (DCB).
A company press release notes that the...
Surmodics announces successful first patient use of Sublime radial access microcatheter
Surmodics has announced that Ankur Lodha, MD, an interventional cardiologist at the Cardiovascular Institute of the South in Lafayette, Lousiana, and Pradeep Nair, director...
Surmodics provides regulatory update related to FDA premarket approval application for...
Surmodics recently announced it has received a letter from the Food and Drug Administration (FDA) related to its premarket approval (PMA) application for the...
SWING trial 12-month data: Novel sirolimus DCB shows ‘great promise’ in...
The Sundance (Surmodics) sirolimus drug-coated balloon (DCB) has an “excellent” safety profile in a “challenging, real-world, predominantly CLTI population,” and has a primary...
SurVeil DCB demonstrates sustained durability of safety, efficacy endpoints in TRANSCEND
In the TRANSCEND clinical trial, the SurVeil drug-coated balloon (DCB; Surmodics) demonstrated "excellent efficacy and safety" out to 24-month follow-up. This is according to...
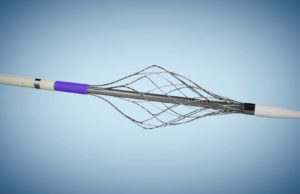
Surmodics announces successful first patient use of Pounce thrombectomy system
Surmodics has announced that J. Michael Bacharach, MD, a vascular interventionalist/cardiologist at North Central Heart, a division of Avera Heart Hospital in Sioux Falls,...
Surmodics builds thrombectomy portfolio with acquisition of Vetex Medical
Surmodics recently announced that it has acquired privately-held Vetex Medical Limited, expanding the company's thrombectomy portfolio with a second Food and Drug Administration (FDA) 510(k)-cleared...
Surmodics announces first patient uses of two Sublime radial access platform...
Surmodics recently announced the successful first uses in patients for two devices within its Sublime radial access platform: the Sublime radial access guide sheath and...