Tag: DCB
Meta-analysis: Drug-coated devices show comparable aggregate outcomes to non-drug-coated devices in...
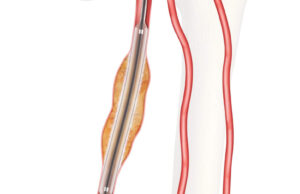
Drug-coated balloon (DCB) angioplasty appears to offer similar benefits as plain old balloon angioplasty (POBA), with or without stenting, for patients with chronic limb-threatening...
FDA grants approval for IDE study on Advance Evero 18 everolimus-coated...
The Food and Drug Administration (FDA) has granted approval for Cook Medical to initiate an investigational device exemption (IDE) study on the Advance Evero...
TCT 2024: One-year data from SIRONA trial show similar results for...
Data from the prospective, multicentre, investigator-initiated SIRONA randomised clinical trial demonstrated MagicTouch (Concept Medical) sirolimus-coated balloons to be noninferior compared to paclitaxel-coated balloons with...
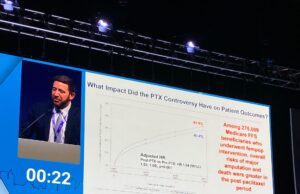
Paclitaxel controversy: Yes, device restrictions did cause harm
“Unfortunately, we are doing worse for our patients today,” were the sobering thoughts of Eric Secemsky (Boston, United States) during a late-breaking presentation in...
The top 10 most popular Vascular Specialist stories of November 2023
In November, the most read stories from Vascular Specialist include a reassessment of which carotid revascularization treatment modality is best after the recent Centers...
Two-year SWING data ‘continue to show promise’ for sirolimus DCB in...
Two-year data from the SWING trial, a first-in-human study of the safety and performance of the Sundance sirolimus drug-coated balloon (DCB; Surmodics), have been...
Popliteal artery lesions: Two-year results from European DCB catheter registry announced
“We face a scarcity of data evaluating endovascular therapy for isolated popliteal artery lesions, known as a difficult vessel bed to treat as we...
Light-activated drug-coated balloon granted FDA approval for clinical study
Alucent Biomedical has announced that the Food and Drug Administration (FDA) granted an investigational device exemption (IDE) for a U.S. clinical study of AlucentNVS,...
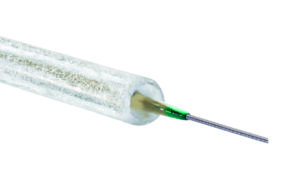
Surmodics receives FDA approval for the SurVeil drug-coated balloon
Surmodics has announced the receipt of Food and Drug Administration (FDA) approval for the SurVeil drug-coated balloon (DCB).
A company press release notes that the...
Surmodics provides regulatory update related to FDA premarket approval application for...
Surmodics recently announced it has received a letter from the Food and Drug Administration (FDA) related to its premarket approval (PMA) application for the...
The top 10 most popular Vascular Specialist stories of December 2022
Among the most-read Vascular Specialist stories in December 2022 were the promising results from a postmarket surveillance study of a paclitaxel DCB; the Society...
Paclitaxel DCB performs well in ‘challenging anatomy’ of femoropopliteal in-stent restenosis,...
The IN.PACT Admiral drug-coated balloon (DCB) shows promising results in treating femoropopliteal in-stent restenosis (ISR) in a Society for Vascular Surgery (SVS) Vascular Quality...
The 10 most popular Vascular Specialist stories of November 2022
Among November's most read Vascular Specialist stories were updates from the BEST-CLI trial in the form of new data and funding; a guest...
Chocolate Touch DCB for treatment of peripheral arterial disease receives FDA...
Genesis MedTech Group announced that the Food and Drug Administration (FDA) has approved the Chocolate Touch drug-coated balloon (DCB) percutaneous transluminal angioplasty (PTA) catheter...
SurVeil DCB demonstrates sustained durability of safety, efficacy endpoints in TRANSCEND
In the TRANSCEND clinical trial, the SurVeil drug-coated balloon (DCB; Surmodics) demonstrated "excellent efficacy and safety" out to 24-month follow-up. This is according to...
Six-month data from SWING below-the-knee, first-in-human trial presented at AMP Europe
Six-month data from the Surmodics SWING first-in-human (FIH) study of the company’s Sundance sirolimus drug-coated balloon (DCB) were shared at the 2022 Amputation Prevention Symposium (AMP) held Oct....
Medtronic gains FDA nod for IN.PACT 018 DCB
Medtronic has announced approval from the Food and Drug Administration (FDA) for the IN.PACT 018 paclitaxel-coated percutaneous transluminal angioplasty (PTA) balloon catheter, a drug-coated...
Two-year Ranger DCB data demonstrate ‘sustained, high rate’ of device efficacy
Patients treated with Boston Scientific’s Ranger drug-coated balloon (DCB) sustained “improved primary patency” with fewer reinterventions than those treated with uncoated devices, two-year results...
VIVA 2021: IN.PACT Admiral DCB found to provide ‘high five-year freedom...
Real-world data drawn from the IN.PACT Global study looking at five-year freedom from clinically driven target lesion revascularization (TLR) among prespecified chronic total occlusion...
FDA official delivers government agency’s early reaction to latest contentious meta-analysis...
HOLLYWOOD, Fla.—A Food and Drug Administration (FDA) official delivered a talk which represents the government agency’s earliest public reaction to the latest meta-analysis suggesting...
Deploying robust, detailed SVS VQI data to help better define role...
In December 2018, the Journal of the American Heart Association published “Risk of death following application of paclitaxel‐coated balloons and stents in the femoropopliteal...
New meta-analysis finds ‘no observed difference’ in mortality between paclitaxel and...
February 2020 brings another paclitaxel device meta-analysis of randomized controlled trials in chronic limb-threatening ischemia (CLTI) patients. Krystal Dinh, BMed, of Westmead Hospital, Sydney,...
Hotly-contested meta-analysis suggests a higher risk of death or amputation at...
A new meta-analysis, just published in the Journal of Vascular and Interventional Radiology (JVIR), suggests significantly reduced amputation-free survival at one year when paclitaxel-coated...