
This advertorial is sponsored by Inari Medical.
Several years ago, when Nicolas Mouawad, MD, division chief of vascular surgery at McLaren Health in Bay City, Michigan, was confronted with an emergency case of deep vein thrombosis (DVT) in which the patient presented with phlegmasia, he faced a dilemma. With the traditional lytic intervention off the table due to the urgent need for an immediate response, Mouawad turned to a new device he hadn’t used before. For the first time, the ClotTriever® System provided Mouawad a DVT intervention procedure with a lytic-free approach in a single-session treatment with wall-to-wall thrombus removal, providing the patient with immediate symptom relief.1
Here, he outlines the current picture of his venous thromboembolism (VTE) practice, how that emergency case helped evolve how he tackles DVTs in appropriate patients, and how he sees the DVT treatment landscape developing going forward.
What does your current venous disease practice look like?
We have a comprehensive venous practice. We manage superficial veins and deep venous disease as well as pulmonary embolism (PE)—the full VTE disease spectrum. We see a significant number of patients who come in with DVT and post-thrombotic syndrome (PTS), as well as with venous leg ulcers, so we feel very comfortable managing the wide array of patients suffering from venous insufficiency and VTE.
Could you expand upon how you go about managing patients with DVT?
Our DVT management is multi-fold. The goal is to, first, relieve the patient’s symptoms, such as significant venous claudication or severe swelling that they find quite bothersome, or pain. Of course, we start with anticoagulation; however, a proportion of patients presenting with this pathology we feel benefit from intervention. Thinking further down the line, we want to avoid patients having significant sequelae of DVT, which, in up to 50% of patients with DVT, means PTS.2 Presently, these PTS patients are treated using the current standard of care, which is anticoagulation and elastic compression stockings. Even though they’re undergoing currently recommended treatments, these patients still develop long-term sequelae of PTS, so our goal here is to help mitigate the possibility of them developing these sequelae.
Can you describe how the ClotTriever first made its way into your toolkit?
Like a lot of other vascular specialists, I was using other devices on the market for the management of patients with DVT. I remember very vividly the first patient I treated with the ClotTriever System presented with phlegmasia. Even though I would consider our traditional treatment algorithm which was thrombolytic therapy, that would not be able to deliver an immediate resolution of the problem. What we needed was an immediate treatment for this patient who essentially had a surgical emergency. My hand was forced to try something that was immediate, single session and able to deliver a result without long infusion times. That case was around six years ago, and, frankly, we haven’t looked back since I started doing this type of treatment.
How does the ClotTriever System differ from other modes of intervention for the treatment of DVT?
Multiple variables of the system are beneficial to me. First, the ClotTriever is a single-session device. Bringing the patient back can be challenging, so a single-session device is important to me. Next, I don’t worry about the chronicity of the thrombus. Patients present within one or two days of discomfort, but we all know that the thrombus has probably been there longer. The symptoms may be acute, but the thrombus itself is likely sub-acute and even older. I have been using the ClotTriever BOLD Catheter to eliminate any guesswork on clot chronicity. Lastly, the unique device design allows me to remove wall-adherent thrombus to minimize residual vein obstruction. In terms of residual vein obstruction, creating more than just a channel with other thrombectomy devices is an important consideration regarding long-term outcomes.
Can you outline ways in which the device has evolved your DVT practice?
There is no associated ICU [intensive care unit] stay like there is when using thrombolytic therapy. There are no associated costs for pharmacologic therapy. There is no need for any capital equipment. Those were the benefits early on. To date, more than 50,000 ClotTriever cases have been performed. Additionally, there exists a plethora of data to support the device. Very aggressive efforts have been made to look at the data. The CLOUT registry is the largest study to assess mechanical thrombectomy to date, with interim two-year outcomes that showed 93% of limbs had none or mild PTS.3 Additionally, my experience—and histological analyses of ClotTriever cases— have not identified any cases where there has been venous valve or vessel wall damage.4 I’ve performed more than 200 DVT cases using the ClotTriever System and seen patients back with marked improvement in their discomfort and Villalta scores for PTS. I don’t think that would have been possible if we were ripping out valves or damaging the vein walls.
What do you think the future holds for DVT treatment?
For me, it’s important to be able to work with the groups investing in data. Unfortunately, the guidelines lag well behind what we see in practice. Of course, that is because a lot of data are necessary before guidelines can be created. Those data are forthcoming, however. The DEFIANCE randomized controlled trial (RCT)—currently the only RCT assessing DVT interventions—will be one such source. DEFIANCE, which compares iliofemoral DVT patients treated with the ClotTriever System versus anticoagulation only, should help support what we see day-to-day in our regular practice.
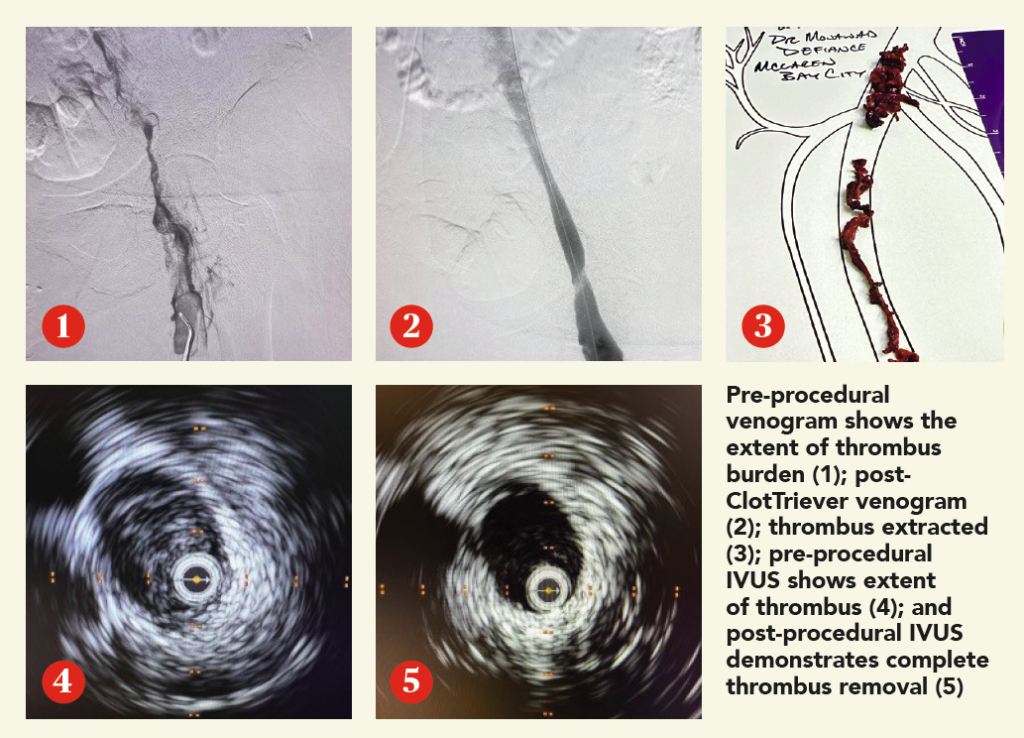 CASE REPORT
CASE REPORT
A woman in her early 60s presented with right leg pain and swelling. An ultrasound revealed unilateral right iliofemoral DVT, and the decision to intervene using the ClotTriever BOLD Catheter was made.
Procedural overview
The right popliteal vein was accessed with a micropuncture needle and wire under ultrasound guidance, and exchanged for a microsheath, ultimately upsizing to a short 10F sheath. A venogram demonstrated significant thrombus within the femoropopliteal segment, common femoral vein and extending into the external iliac vein. The lesion was crossed into the inferior vena cava (IVC). Next, pullback intravascular ultrasound (IVUS) from the IVC to the access site was performed to identify the extent of thrombus. A glidewire was advanced up into the right subclavian vein, and a vertebral catheter was advanced over it. Next, the glidewire was exchanged for a guidewire 7cm tip. A 19F dilator was used, followed by insertion of the ClotTriever sheath. The funnel was deployed under fluoroscopic guidance and the ClotTriever BOLD Catheter was advanced into the popliteal vein. The nitinol coring element and mesh collection bag were then deployed at the iliac vein, and the catheter was then retracted, capturing and removing significant acute thrombus. In total, four ClotTriever BOLD passes were completed. Completion IVUS showed complete thrombus removal. Completion venogram demonstrated brisk cephalad flow. Total procedure time was 35 minutes, while total device time was 10 minutes. There was estimated total blood loss of 20ml.
Conclusion
The patient’s pain resolved immediately postprocedure, with the swelling improving greatly. In follow-up, the swelling was resolved and the patient is doing well.
References
- Dexter D et al. JSCAI. 2024.
- Kahn SR, et al. J Thomb Thrombolysis. 2016.
- Dexter D. Interim two-year outcomes from the fully enrolled CLOUT registry. AVF. 2024.
- Silver MJ, et al. Catheter Cardiovasc Interv. 2021.
The ClotTriever Thrombectomy System is indicated for (1) the non-surgical removal of thrombi and emboli from blood vessels; and (2) injection, infusion and/or aspiration of contrast media and other fluids into or from a blood vessel. The ClotTriever Thrombectomy System is intended for use in the peripheral vasculature, including deep vein thrombosis (DVT). Refer to IFU for complete indications for use, contraindications, warnings and precautions. Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. All trademarks are property of their respective owners.












