Thirty-day results from two trials assessing the performance of investigational carotid artery stenting systems—the Neuroguard and the CGuard—were recently presented at VIVA 2023.
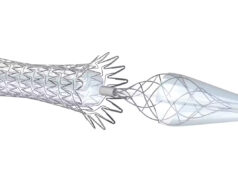
Stenting with the CGuard embolic protection carotid stent system (InspireMD) in patients with carotid artery stenosis and at high risk for carotid endarterectomy (CEA) had a death (all-cause mortality), any stroke or myocardial infarction rate of 0.95% from procedure through 30 days of follow-up, reported D. Christopher Metzger, MD, an interventional cardiologist at OhioHealth in Columbus, Ohio, at the Las Vegas meeting (Oct. 30–Nov. 2).
The C-GUARDIANS U.S. investigational device exemption (IDE) pivotal trial looked at 316 patients from July 2021–June 2023 who were prospectively enrolled in the single-arm study performed at 24 sites in the U.S. and Europe. The primary endpoint was a composite of either incidence of major adverse events including death (all-cause mortality), any stroke or myocardial infarction through 30 days post-index procedure; or ipsilateral stroke from day 31 to day 365 post-procedure.
InspireMD anticipates reporting primary endpoint results from C-GUARDIANS—which the company stated may support a premarket approval (PMA) application to the Food and Drug Administration (FDA)—in the second half of 2024.
In the PERFORMANCE II prospective, multicenter study evaluating the safety and effectiveness of the Neuroguard integrated embolic protection (IEP) system, the reported 30-day stroke rate was 1.31% in the intention-to-treat analysis and 0.98% in a per-protocol analysis, with no major strokes or contralateral strokes, and all patients returning to baseline neurologically within 30 days, William Gray, MD, the system chief of cardiovascular disease at Main Line Health in Philadelphia, told VIVA 2023.
The Neuroguard (Contego Medical) PERFORMANCE II single-arm study is assessing the device among 305 patients at 40 clinical sites in the U.S. and Europe. At one-year follow-up (all stroke within 30 days, and ipsilateral stroke between day 31 and 12 months), the reported stroke rate was 1.68% in the intention-to-treat analysis and 1.35% in a per-protocol analysis, Gray added. No major strokes or neurological deaths occurred in the study.
In addition to the PERFORMANCE II study—in which the Neuroguard was placed via transfemoral or transradial access—the PERFORMANCE III study is currently enrolling patients to evaluate the same stent placed via direct transcarotid access, Contego Medical reported.