Results from a multicenter randomized controlled trial (RCT) indicate that pre-emptive inferior mesenteric artery (IMA) embolisation during endovascular aneurysm repair (EVAR) does not significantly reduce aneurysm sac volume or rates of type II endoleak.
The CLARIFY IMA study—which the authors note is the first multicenter RCT to provide data on the effectiveness of pre-emptive IMA embolisation during EVAR—was recently published in the European Journal of Vascular and Endovascular Surgery (EJVES).
Opening their paper, Shigeo Ichihashi (Nara Medical University, Nara, Japan) and colleagues write that persistent type II endoleak following EVAR has been identified as a cause of aneurysm sac expansion, which they note can lead to reintervention and aneurysm rupture.
They go on to state that the role of pre-emptive IMA embolisation to prevent type II endoleak and sac expansion “remains controversial,” with the present study aiming to evaluate the influence of IMA embolisation on aneurysm sac change after EVAR.
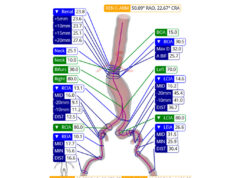
In the CLARIFY IMA study, which was conducted at 24 centres in Japan, patients with a fusiform abdominal aortic aneurysm (AAA) were randomised to undergo EVAR either with or without pre-emptive IMA embolisation. The primary outcome was defined as the percentage change in computed tomography (CT)-assessed aneurysm sac volume at 12 months, with secondary outcomes including sac diameter changes, prevalence of type II endoleak, freedom from reintervention, and overall survival at six, 12, and 24 months.
Ichihashi and colleagues share that 138 patients with AAA (mean age, 76 years; 117 men) were randomised to either the IMA embolisation (n=70) or the control group (n=68). Of the 70 patients included in the former, the authors report that IMA embolisation was successful in 63 (90%) patients.
The authors add that, at 12 months, there was no statistically significant difference in aneurysm sac volume change between the embolisation group and control group. Furthermore, no statistically significant differences were observed in sac diameter change, rate of type II endoleak, freedom from reintervention, and overall survival at any follow-up time point.
“Unlike previous single-centre RCTs, the findings indicate that IMA embolisation does not significantly reduce aneurysm sac volume or rates of type II endoleak,” Ichihashi and colleagues conclude. “These results suggest that while safe and technically feasible, pre-emptive IMA embolisation may not be effective in EVAR procedures, prompting further investigation into alternative methods for reducing endoleak and sac expansion in clinical practice.”
Ichihashi presented these findings during the Janet Powell session on late-breaking news in clinical trials at the 39th European Society for Vascular Surgery (ESVS) annual meeting (23–26 September, Istanbul, Türkiye). Following his talk, one question from the audience probed possible reasons behind the results.
“Can I ask why you think you did not show a difference?” Richard Bulbulia (University of Oxford, Oxford, UK) posed. “Was your study too small, or was your study too short, or perhaps both were true?”
“I believe two years is long enough,” Ichihashi responded. In their EJVES paper, the authors elaborate on the length of follow-up during discussion of several study limitations. They acknowledge that the follow-up period of 24 months, “while sufficient to observe initial trends,” may not have captured sac behaviour or aneurysm-related death in the long term.
Among other limitations, the authors highlight the fact that the COVID-19 pandemic prevented them from recruiting as many patients to the trial as planned, the incomplete data regarding type II endoleak during follow-up for approximately one-third of the participants who underwent only plain CT, and the possibility that the sample size “may not have been large enough to detect smaller but clinically meaningful differences between the groups”.