Royal Philips today announced the first implant of the Duo venous stent system, indicated to treat symptomatic venous outflow obstruction in patients with chronic venous insufficiency (CVI), following premarket approval (PMA) from the Food and Drug Association (FDA).
On June 11, Erin Murphy, MD, a vascular surgeon and director of the Venous and Lymphatic Program at the renowned Atrium Health’s Sanger Heart & Vascular Institute in Charlotte, North Carolina, and an investigator in the VIVID study, successfully used the Duo stent for the first time outside of a clinical trial.
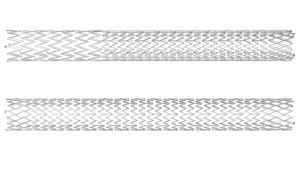
The Duo system is comprised of two stents—Duo Hybrid and Duo Extend—of various sizes. Duo Hybrid has a distinct integrated design that combines multiple zones of differing mechanical properties into a single stent. For long lesions, Duo Extend smoothly overlaps with the Duo Hybrid to extend therapy. These two stents are designed to work together and minimize the risk of stent fracture and corrosion, while providing an option to stent within caudal veins with smaller diameters.
“Duo is the first stent that offers a differential design for the challenges of venous anatomy—a focal area that withstands the forces of compression as well as the flexibility to accommodate curvature of the vessel,” said Kush Desai, MD, an interventional radiologist and associate professor of radiology, surgery and medicine at Northwestern University in Chicago, and also a VIVID study investigator.
The VIVID study is a global, prospective, multi-center, single-arm, non-blinded clinical trial conducted in the U.S. and Poland, evaluating the safety and efficacy of the Duo stent in the treatment of nonmalignant iliofemoral occlusive disease. It enrolled 162 subjects at 30 centers with three patient populations—non-thrombotic iliac vein lesion (NIVL), post-thrombotic syndrome (PTS) and acute deep vein thrombosis (aDVT). The VIVID study is now in 36-month follow-up and, upon FDA PMA approval, transitioned from an investigational device exemption (IDE) study to a post-approval study.
The VIVID study met all of its primary safety and efficacy performance goals. The 12-month effectiveness endpoint for primary patency reached 90.2%, which exceeded the performance target goal of 77.3%. The 12-month primary safety endpoint of 98.7% also exceeded the corresponding performance goal of 89%.
In addition, quality of life and venous functional assessments that were performed in the VIVID study—including Clinical-Etiology-Anatomy-Pathophysiology (CEAP), Venous Clinical Severity Score (VCSS), Villalta, EQ-5D-3L and VEINES scores—showed sustained improvements compared to baseline at 12 months.
The VIVID study was the first clinical trial to mandate IVUS use to aid in lesion assessment and stent sizing prior to device implantation. According to prior published research, IVUS supports accurate diagnosis of venous disease and has been shown to change 57% of treatment plans compared to venography alone.