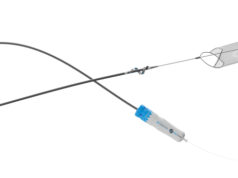
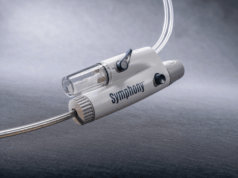
Imperative Care has announced the completion of patient enrollment in its SYMPHONY-PE study, a pivotal investigational device exemption (IDE) trial evaluating the safety and efficacy of the company’s Symphony thrombectomy system for the treatment of acute pulmonary embolism (PE).
Imperative Care has announced the completion of patient enrollment in its SYMPHONY-PE study, a pivotal investigational device exemption (IDE) trial evaluating the safety and efficacy of the company’s Symphony thrombectomy system for the treatment of acute pulmonary embolism (PE).
Designed to expand Symphony’s current indication for use to include PE, the study was conducted at 19 leading interventional radiology, interventional cardiology and vascular surgery centers across the USA.
“While progress has been made in the treatment of pulmonary embolism, there is still a need for therapies that may offer improvements in safety, efficiency and long-term patient outcomes,” said interventional radiologist Vivian L. Bishay, from Mount Sinai Health System in New York City, and national co-principal investigator of the SYMPHONY-PE study. “This study represents a promising step forward in evaluating next generation technology to expand treatment options for physicians and their patients.”