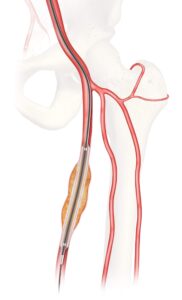
The Food and Drug Administration (FDA) has granted approval for Cook Medical to initiate an investigational device exemption (IDE) study on the Advance Evero 18 everolimus-coated percutaneous transluminal angioplasty (PTA) balloon catheter, the company reported today.
The clinical study will assess the safety and effectiveness of the Evero drug-coated balloon (DCB) when compared to commercially available paclitaxel-coated balloons for the treatment of peripheral artery disease (PAD). Currently there are no everolimus-coated balloon devices commercially available in the U.S., and this IDE study is the first U.S. head-to-head evaluation of everolimus- and paclitaxel-coated balloons to treat lesions of the superficial femoral and popliteal arteries, according to Cook.
The EVERO trial is a prospective, U.S. multicenter, stratified, blinded, randomized control trial (RCT) with a parallel pharmacokinetic (PK) study. Cook intends to enroll 410 patients in the pivotal trial and 30 patients in the PK evaluation. The primary safety endpoint is a composite of freedom from device- or procedure-related death at 30 days, freedom from target limb major amputation at 12 months, and from target lesion revascularization (TLR) at 12 months. The primary effectiveness endpoint is primary patency, defined as peak systolic velocity ratio (PSVR) ≤2.4 at 12 months and freedom from clinically driven TLR (CD-TLR) at 12 months.











