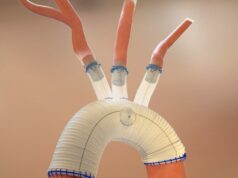
Endospan shared 30-day results from the first 22 patients enrolled in the TRIOMPHE investigational device exemption (IDE) study in a late-breaking presentation at the 60th annual meeting of the Society of Thoracic Surgeons (27–29 January, San Antonio, USA).
TRIOMPHE is a multi-arm, multicentre, non-randomised, prospective clinical study to evaluate the safety and effectiveness of Nexus in treating thoracic aortic lesions involving the aortic arch. The study will enrol 110 patients at up to 31 sites.
“We were pleased to see that in this high-risk patient population, the 30-day data for these first 22 patients showed a low mortality rate, no disabling strokes, paraplegia, or renal failure and short ICU [intensive care unit] and hospital lengths of stay,” said Bradley Leshnower, director of thoracic aortic surgery at Emory University School of Medicine (Atlanta, USA). “One year data are pending.”
A press release details that more than 120,000 patients suffer thoracic aortic arch disease every year in the USA and Europe, with only about 25% diagnosed or treated. Despite significant advancements, open surgical aortic arch repair maintains high mortality and morbidity. Patients with excessive perioperative risk or anatomical factors are not indicated for surgery, yet anatomical complexity and lack of approved devices for the arch has often prohibited endovascular repair. This makes the choice of treatment difficult or even impossible for some patients. Providing the alternative of minimally invasive repair decreases the requirement for extra corporal circulation and possibility of hypothermia, translating into reduced procedure and hospitalisation time.
“Nexus is designed for total endovascular arch repair to address the specific challenges of the aortic arch anatomy,” said Kevin Mayberry, Endospan CEO. “We are pleased to see that these early data align with the results achieved during the EU clinical study. These data suggests that NEXUS may provide surgeons with a straightforward, minimally invasive solution for aortic arch repair that allows for procedural consistency with reliable patient outcomes.”