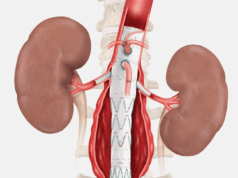
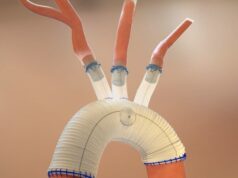
Endospan today announced the completion of enrolment for the primary arm of its TRIOMPHE investigational device exemption (IDE) clinical study investigating the Nexus aortic arch stent graft. The study is evaluating the safety and efficacy of the device for the treatment of aortic arch disease.
A company press release details that the Nexus aortic arch stent graft is a bi-modular off-the-shelf device designed to provide a minimally invasive solution for patients with aortic arch disease. The TRIOMPHE IDE study is a three-arm non-randomised study conducted at 30 aortic centres across the USA and one centre in New Zealand, enrolling patients with a variety of aortic arch pathologies.
“We are thrilled to announce the completion of enrolment for the primary arm of this important clinical study,” said Kevin Mayberry, chief executive officer of Endospan. “The Nexus aortic arch stent graft has the potential to significantly improve outcomes for patients with aortic arch disease. We are committed to bringing this innovative technology, which is already a proven platform in Europe, to the USA as quickly as possible.”
Brad Leshnower (Emory School of Medicine, Atlanta, USA), who is the cardiac national principal investigator of the study, added: “We are excited to be part of this groundbreaking study. While the early results from the TRIOMPHE study presented at STS this year suggest that the Nexus system can be used safely to treat aortic arch disease in a high-risk surgical cohort with a low rate of stroke, we anxiously await the results from the full cohort.”
Ross Milner (UChicago Medicine, Chicago, USA), who is the vascular national principal investigator, commented: “The Nexus device has the potential to revolutionise the treatment of aortic arch disease by offering a less invasive alternative to open surgery. We are eager to see the results of this study regarding midterm durability and patient outcomes.”
The company states that it plans to monitor the patients’ safety and efficacy for one year prior to submitting for US Food and Drug Administration (FDA) approval.