For University of Calgary, Alberta, Canada, vascular surgeon Randy Moore, MD, the route to a world of precision care for individual abdominal aortic aneurysm (AAA) patients—involving aortic wall strength-mapping technology developed over the course of the last 15 years, allied to an artificial intelligence (AI)-powered algorithm—is drawing nearer. Right now, says the associate professor of vascular and endovascular surgery, the decision to treat AAAs is based on a single diameter measure derived from population-based information. “That is flawed,” he implored. “And this hasn’t changed in over half a century.”
Moore was speaking during the 2023 Houston Aortic Symposium (March 16–18), held in Houston, Texas, where he delivered insight into RAW (Regional Areas of Weakness) Maps and its aim to provide a precision medicine solution for AAA patients. “When we look at size alone as a measure for patients at risk of aortic aneurysm disease, we ignore all these other things that are critical to our decision-making,” he told those gathered for a talk on the ViTAA Medical Solutions technology. Moore is the company’s medical director and a co-founder, and it is on his clinical experience that early testing of the tool’s effectiveness is based.
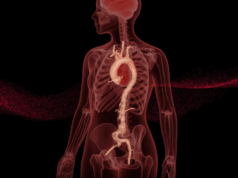
Moore emphasized the core principle behind AI in clinical work: analyzing large amounts of data, recognizing patterns, and predicting outcomes from pattern-recognition algorithms. “In our center, for the past 15 years, we have developed a number of algorithms that allow us to link actual tissue strength to risk in terms of wall strength analysis,” he said.
The ViTAA solution he proposes uses a RAW mapping score of aortic wall tissue integrity so that clinicians can talk through with patients the risk associated with their condition. “We knew our mapping technology was very effective at identifying wall strength and weakness,” he said. “But is that map a useful tool to allow for forward prediction—not just a map in real time but the ability to move ahead and provide the clinician with a tool?”
An initial pilot study of 36 patients showed a statistically significant correlation between ViTAA’s RAW Maps and aortic growth over time. “We could, from a single ViTAA analysis, identify or predict risk in that patient moving forward up to 12 months with a statistically significant outcome,” Moore said. “We knew the mapping tool was good, but we wanted to understand how the addition of artificial intelligence would unlock, not just that mapping technique, but the predictive value moving forward.”
That’s where the ViTAA Aortic Model comes in, he continued. This involves the use of patient imaging—which the system “flattens out,” gathering 3,000 coordinates of the aorta—and comparisons between different aortic scans at “exactly the part of the aortic wall we want to address.” The model enables serial investigations, Moore said, and using explainable AI—unroofing the concept of black-box AI—clinicians can then see what’s being measured and modified for auditable purposes.
“Our vision is that with the AI we have combined with the mapping technology, we are going to be able to provide the clinician with a go-forward view of a patient’s down-the-road behavior, which can then inform current real-time decision-making.”
Of course, the AI algorithm requires datasets from which to learn, Moore conceded. As with human facial recognition technology that generates people who do not exist, artificial patients, too, can be created. “Why would we do this?” asked Moore.
In order to generate a large enough dataset so that the researchers can get the answers they need in a short period of time, he said. “You can generate human aortic characteristics without actually including any backtraceable, real patient data. How does this work? You take real patient data, feed it in, and a generative adversarial network then creates this synthetic dataset that you can learn to train your AI.”
Moore pointed to a concrete use case his team developed. “In our initial 16-patient pilot study looking at the strength of the aortic neck, we were able to identify a statistically significant relationship to a weak neck and subsequent type 1a endoleak. But if you look at the dataset, it was heavily weighted toward endoseal, with a small number of endoleaks. To make that learning set more applicable, we then created a synthetic database with 5,000 patients, with equipoise between the two groups. That took six minutes on a laptop.” After an analysis probing the synthetic database’s validity, the researchers found that it still produced what is considered a “very excellent” predictive rate.
Moore and colleagues are now focused on validating the model through a North American multi-site registry and a number of research-use only sites accumulating datasets on aortic pathology. “The future of precision aortic care ain’t what it used to be as some of these tools continue to roll out.”