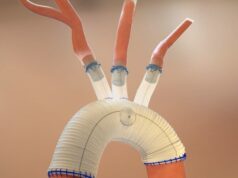
Artivion recently announced that the Food and Drug Administration (FDA) has granted a humanitarian device exemption (HDE) for use of the AMDS hybrid prosthesis (formerly known as the Ascyrus Medical dissection stent) in acute DeBakey type I dissections in the presence of malperfusion.
A press release notes that the AMDS is the world’s first aortic arch remodeling device for use in the treatment of acute DeBakey type I aortic dissections.
An HDE is a marketing application for a product that has been designated a humanitarian use device (HUD). Artivion details that AMDS received both HUD and breakthrough designation, due to its intended benefit for patients in the treatment or diagnosis of a rare disease or condition in which no other comparable options currently exist. The HDE allows for commercial distribution of AMDS in the U.S. prior to anticipated approval of a premarket approval (PMA) application.
Under the HDE, AMDS will be available as a treatment for acute DeBakey type I dissections in the presence of malperfusion, which represent approximately 40% of all acute DeBakey type I dissections in the U.S. The PMA, if approved, is expected to cover all acute DeBakey type I dissections with and without malperfusion, representing an estimated US$150 million annual US market opportunity.
The HDE for AMDS was granted following the availability of full cohort data from the PERSEVERE US investigational device exemption (IDE) trial for AMDS. The trial consisted of 93 participants in the U.S. and met its primary endpoints demonstrating significant reduction of major adverse events (MAEs), including all-cause mortality, stroke, renal failure requiring dialysis, and myocardial infarction at 30 days following AMDS implantation.
More specifically, data showed, from the use of AMDS, a 72% reduction in all-cause mortality and a 54% reduction in primary MAEs, with zero occurrence of distal anastomotic new entry (DANE), when compared to the current standard of care hemiarch procedure. Wilson Szeto, chief of cardiovascular surgery at Perelman School of Medicine at the University of Pennsylvania in Philadelphia recently presented the data from the PERSEVERE US IDE trial as a late-breaking abstract at the Society of Thoracic Surgeons (STS) annual meeting.