This advertorial is sponsored by Shockwave Medical.
Paul J. Foley III, MD, a vascular surgeon and director of the non-invasive vascular lab at Doylestown Hospital in Doylestown, Pennsylvania, and adjunct associate professor of surgery at the University of Pennsylvania in Philadelphia, discusses the virtues of the new Shockwave E8 IVL catheter.
Endovascular interventions for chronic limb-threatening ischemia (CLTI) are technically demanding endeavors. Multi-level arterial disease, long-length lesions and other complex plaque characteristics are frequently encountered when treating these patients.1,2
Calcium modification with intravascular lithotripsy (IVL) has emerged as a useful tool to combat the challenges of calcified lesions with the goal of maximizing luminal gain to achieve improved endovascular outcomes, both radiographically and clinically.³ Balanced lithotripsy pulse delivery across longer-length lesions, as well as effectively treating calcified disease across multiple arterial beds, can be laborious considering the number of available pulses and the length of the IVL catheter relative to the extent of disease.
To address this, the Shockwave IVL peripheral portfolio has now been enhanced with the addition of the Shockwave E8 catheter. The Shockwave E8 contains eight emitters across an 80mm-length balloon platform with treatment diameters ranging from 2.5–6mm and the ability to deliver up to 400 pulses. A working length of 150cm now provides an extended reach for more distal disease. The Shockwave E8 catheter allows for expanded application of IVL in treating a wide range of infrainguinal disease. Longer-length lesions involving the superficial femoral (SFA) and popliteal arteries or disease involving multiple tibial arteries that may have previously required a very selective pulse delivery approach can now be more broadly treated with the Shockwave E8.
Case report
The following case highlights the versatility of the Shockwave E8 IVL catheter to treat long-length lesions in two tibial arteries.
History
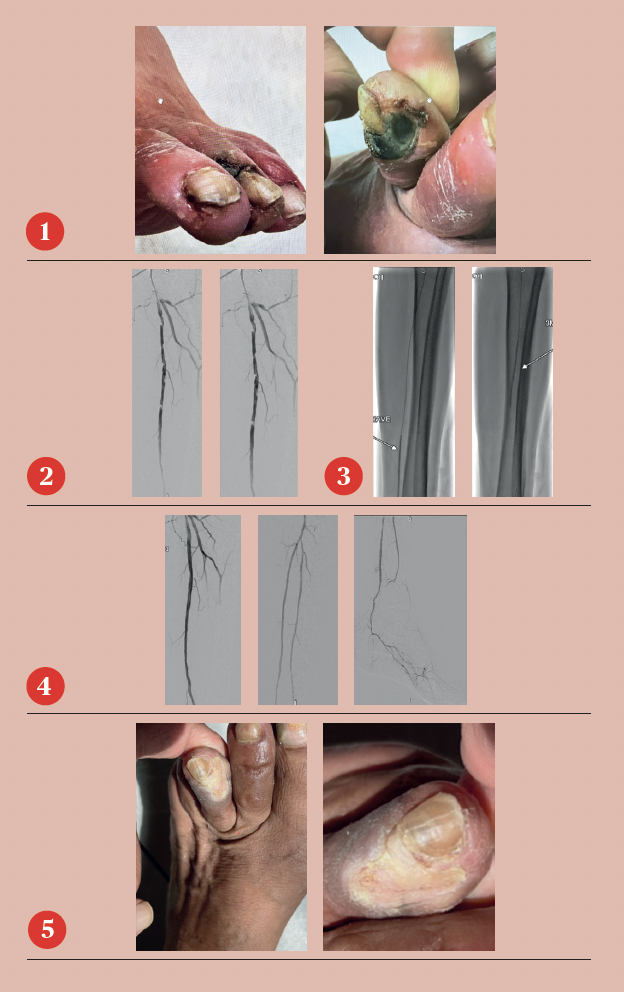
A 78-year-old man who resides in Uzbekistan presented to the office with CLTI of the left lower extremity manifested by a non-healing gangrenous left toe ulcer and associated rest pain (Figure 1). He was initially evaluated by providers in his home country and had a recent arteriogram in Uzbekistan demonstrating severe multi-level arterial disease in the left lower extremity, but no intervention was performed. The patient’s son, who lives in the U.S., brought him to the office for vascular surgery evaluation. The patient’s past medical history is notable for hypertension, hypercholesterolemia and a heavy smoking history. On physical examination, he had a palpable left femoral pulse but non-palpable pedal pulses in the left foot.
Preoperative ankle brachial index (ABI) of the left lower extremity was severely reduced at 0.31 and the left toe pressure was zero. He was taken to the operating room for a left lower extremity arteriogram and endovascular intervention.
Arteriogram, left lower extremity
An arteriogram of the left lower extremity was performed via antegrade left common femoral artery access based on preoperative review of the lower extremity arteriogram from Uzbekistan. Scattered non-calcified plaque with moderate to high-grade stenoses were identified in the proximal and mid superficial femoral artery. Diffuse severe calcified tibial artery disease was identified. There was a long segment stenosis of the proximal and mid posterior tibial artery coupled with a more distal occlusion. The proximal peroneal artery was calcified and occluded with distal reconstitution identified. There was complete occlusion of the anterior tibial artery (Figure 2).
Shockwave E8 3mm x 80mm to the posterior tibial artery
The posterior tibial artery was successfully crossed first. The long-length posterior tibial artery disease was treated with a Shockwave E8 3.0mm x 80mm IVL catheter, with no pre-dilatation required. A total of 200 pulses were delivered along the length of the posterior tibial artery (Figure 3).
Shockwave E8 3mm x 80mm to the peroneal artery
Following this, the peroneal artery occlusion was successfully crossed. The long-length peroneal artery disease was treated with the same Shockwave E8 3mm x 80mm IVL catheter also with no pre-dilatation required. The remaining 200 pulses were delivered across the length of the peroneal artery disease (Figure 3).
Left SFA intervention
To ensure adequate inline blood flow to the tibial vessels, the SFA disease was treated with drug-coated balloon angioplasty, followed by self-expanding stent placement.
Post-intervention arteriogram
An excellent technical result was achieved. The SFA was widely patent with no significant residual stenosis. The posterior tibial and peroneal arteries were widely patent with brisk flow and no residual stenosis. Significantly improved flow was identified to the foot (Figure 4).
Post-intervention follow-up
The patient had an uneventful postoperative recovery. His post-intervention lower extremity arterial studies demonstrated a significant improvement in both the ABI and toe-brachial index (TBI). At his one-month postoperative visit, he had completely healed the toe ulcer without any further intervention (Figure 5).
References
- Fitzgerald PJ et al. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation, 1992
- Rocha-Singh KJ et al. Peripheral arterial calcification: Prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv, 2014
- Armstrong E. VIVA Late Breaking Clinical Trial, 2022
Paul J. Foley III is a paid consultant of Shockwave Medical.
Peripheral Important Safety Information In the United States: Rx only. Indications for Use—The Shockwave Medical Intravascular Lithotripsy (IVL) System is intended for lithotripsy-enhanced balloon dilatation of lesions, including calcified lesions, in the peripheral vasculature, including the iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries. Not for use in the coronary, carotid or cerebral vasculature Contraindications—Do not use if unable to pass 0.014” (M5, M5+, S4, E8) or 0.018” (L6) guidewire across the lesion-Not intended for treatment of in-stent restenosis or in coronary, carotid, or cerebrovascular arteries. Warnings—Only to be used by physicians who are familiar with interventional vascular procedures—Physicians must be trained prior to use of the device— Use the generator in accordance with recommended settings as stated in the Operator’s Manual. Precautions—use only the recommended balloon inflation medium—Appropriate anticoagulant therapy should be administered by the physician—Decision regarding use of distal protection should be made based on physician assessment of treatment lesion morphology. Adverse effects—Possible adverse effects consistent with standard angioplasty include—Access site complications— Allergy to contrast or blood thinner. Arterial bypass surgery—Bleeding complications—Death— Fracture of guidewire or device—Hypertension/Hypotension— Infection/sepsis—Placement of a stent—renal failure—Shock/ pulmonary edema—target vessel stenosis or occlusion— Vascular complications. Risks unique to the device and its use—Allergy to catheter material(s)—Device malfunction or failure—Excess heat at target site. Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions and adverse events. www.shockwavemedical.com/IFU. SPL-73151 Rev. A