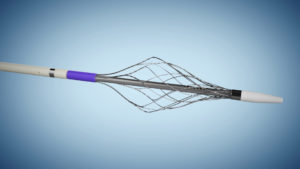
Surmodics recently announced that it has acquired privately-held Vetex Medical Limited, expanding the company’s thrombectomy portfolio with a second Food and Drug Administration (FDA) 510(k)-cleared device, the ReVene thrombectomy catheter.
The ReVene mechanical thrombectomy catheter is specifically designed to remove large, mixed-morphology blood clots commonly found with venous thromboembolism (VTE). According to a press release, the device’s dual action technology efficiently removes mixed-morphology clot in a single session, minimizing the need for thrombolytics and without capital equipment.
“The ReVene thrombectomy catheter has the potential to significantly expand the use and accessibility of venous mechanical thrombectomy by allowing physicians to intervene early and complete the procedure in a single session,” said Stephen Black, MD, consultant vascular surgeon at Guy’s and St Thomas’ NHS Foundation Trust, London, England, principal investigator and leading enroller of the VETEX feasibility study. “The ease of use, intuitive design, and efficient performance of this device enables it to become the first-line treatment and a confident choice by venous interventionalists.”
Under the terms of the acquisition agreement, Surmodics acquired Vetex with an upfront payment of $39.9 million. Additional payments of up to $7 million, $3.5 million of which are guaranteed, may be made upon achievement of certain product development and regulatory milestones.
Surmodics expects to initiate clinical evaluation activities for the Pounce arterial thrombus retrieval system for removing clot in peripheral arteries in the second half of fiscal year 2021 and for the ReVene thrombectomy catheter for removal of clot from veins in fiscal year 2022. A projected timeline for further commercialization will be announced later this fiscal year.











