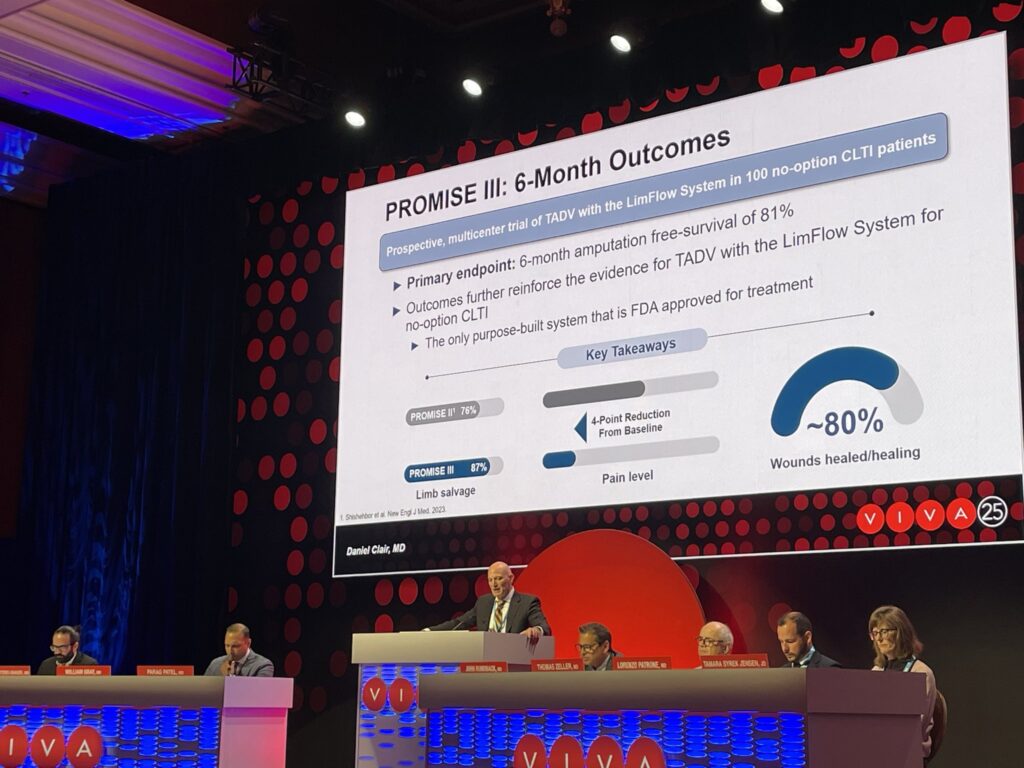
Six-month outcomes among no-option chronic limb-threatening ischemia (CLTI) patients treated using transcatheter arterialization of the deep veins with the LimFlow system (Stryker/Inari Medical) in the PROMISE III trial showed a 81% rate of amputation-free survival.
Principal investigator Daniel Clair, MD, chair of vascular surgery at Vanderbilt University in Nashville, Tennessee, delivered the primary safety endpoint result from PROMISE III at at the 2025 Vascular Interventional Advances (VIVA) conference (Nov. 2–5) in Las Vegas. He told attendees that the six-month data for the “only purpose-built system that is FDA [Food and Drug Administration]-approved for treatment” of no-option CLTI “reinforce the evidence for TADV with the LimFlow system” among this patient group.
A total of 100 patients (103 limbs) underwent TADV between 2022 and 2024 in PROMISE III. All included limbs had non-healing ulcers or gangrene, with 75% Rutherford class 5 and 25% class 6. Technical success was 97%.
At six months, limb salvage was 87%, survival was 93.8%, and approximately 80% of wounds were completely healed or healing, Clair added. The median pain score was 2, “significantly decreased” from 6 at baseline (p<0.0001).











