Tag: Terumo Aortic
Terumo Aortic announces launch of Fenestrated Treo pivotal IDE study in...
Terumo Aortic today announced enrollment of the first patient in the Fenestrated Treo pivotal investigational device exemption (IDE) study in the U.S. This study...
Terumo Aortic and Bentley announce collaboration on US FEVAR study
Terumo Aortic and Bentley today announced their partnership in a clinical study in the USA. The objective is to obtain US Food and Drug...
Terumo Aortic announces ‘significant policy milestone’ with CMS establishing new DRG...
Terumo Aortic today announced what it describes as a "significant policy milestone" with the Centers for Medicare & Medicaid Services (CMS) establishing a new...
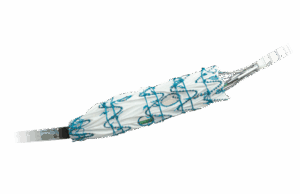
FDA approves dissection and transection indication expansion for RelayPro stent graft...
Terumo Aortic announced that the Food and Drug Administration (FDA) has granted approval of the RelayPro thoracic stent graft device for the treatment of...
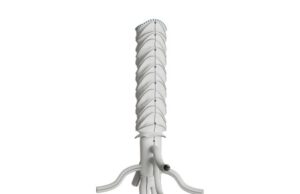
First implant of custom-made thoracoabdominal hybrid device in North America announced
Terumo Aortic announced the first North American implant of a custom-made hybrid device, Thoracoflo.
A press release noted that the device is used to treat...
Terumo Aortic announces new technology add-on payment for Thoraflex Hybrid device...
The Centers for Medicare and Medicaid Services (CMS) has granted approval of a new technology add-on payment (NTAP) for Terumo Aortic’s Thoraflex Hybrid device—used...
Terumo Aortic announces first commercial implant of Thoraflex Hybrid in US
Following the recent approval by the Food and Drug Administration (FDA) of the Thoraflex Hybrid frozen elephant trunk (FET) device for the treatment of...
Terumo Aortic announces first commercial implants of RelayPro in US
Terumo Aortic today announced the first commercial implants in the U.S. of its RelayPro thoracic stent-graft system. This follows the recent approval by the...
RelayPro thoracic stent graft approved by FDA
Terumo Aortic today (Friday, Aug. 6) announced that the Food and Drug Administration (FDA) has granted approval of the RelayPro thoracic stent-graft system for...
RelayPlus thoracic stent graft post-approval study reports low operative mortality
Terumo Aortic has announced the midterm results from the RelayPlus thoracic stent graft system post-approval study, revealing low operative mortality and morbidity— supporting its...