Tag: intervene
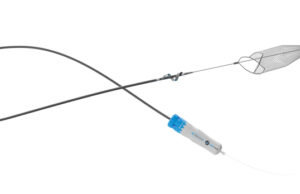
InterVene receives US FDA 510(k) clearance for Recana thrombectomy system
InterVene recently announced it has received 510(k) clearance from the US Food and Drug Administration (FDA) for the Recana thrombectomy catheter system, which is...
First patient treated with InterVene’s Recana thrombectomy catheter system
InterVene announced today that the first patient has been treated with its Recana thrombectomy catheter system for venous in-stent restenosis (ISR).
The company shares...