Tag: EU MDR
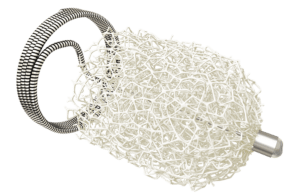
MDR certification secured for Impede embolization plug devices
Shape Memory Medical announced that its Impede embolization plug product family has received certification as a Class III device under the European Union (EU)...
Medical Device Regulation goes into effect across EU
The European Union (EU) Medical Device Regulation (MDR) takes effect from today (May 26).
The Regulation revises quality and safety standards and the range of...